Voltage sensitivity and gating charge in Shaker and Shab family potassium channels
- PMID: 10539976
- PMCID: PMC2230542
- DOI: 10.1085/jgp.114.5.723
Voltage sensitivity and gating charge in Shaker and Shab family potassium channels
Abstract
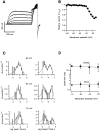
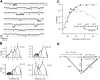
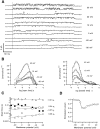
The members of the voltage-dependent potassium channel family subserve a variety of functions and are expected to have voltage sensors with different sensitivities. The Shaker channel of Drosophila, which underlies a transient potassium current, has a high voltage sensitivity that is conferred by a large gating charge movement, approximately 13 elementary charges. A Shaker subunit's primary voltage-sensing (S4) region has seven positively charged residues. The Shab channel and its homologue Kv2.1 both carry a delayed-rectifier current, and their subunits have only five positively charged residues in S4; they would be expected to have smaller gating-charge movements and voltage sensitivities. We have characterized the gating currents and single-channel behavior of Shab channels and have estimated the charge movement in Shaker, Shab, and their rat homologues Kv1.1 and Kv2.1 by measuring the voltage dependence of open probability at very negative voltages and comparing this with the charge-voltage relationships. We find that Shab has a relatively small gating charge, approximately 7.5 e(o). Surprisingly, the corresponding mammalian delayed rectifier Kv2.1, which has the same complement of charged residues in the S2, S3, and S4 segments, has a gating charge of 12.5 e(o), essentially equal to that of Shaker and Kv1.1. Evidence for very strong coupling between charge movement and channel opening is seen in two channel types, with the probability of voltage-independent channel openings measured to be below 10(-9) in Shaker and below 4 x 10(-8) in Kv2.1.
Figures
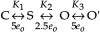




Similar articles
-
Role of the S2 and S3 segment in determining the activation kinetics in Kv2.1 channels.J Membr Biol. 2001 Jul 1;182(1):49-59. doi: 10.1007/s00232-001-0029-x. J Membr Biol. 2001. PMID: 11426299
-
Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation.J Gen Physiol. 1999 Mar;113(3):389-414. doi: 10.1085/jgp.113.3.389. J Gen Physiol. 1999. PMID: 10051516 Free PMC article.
-
Voltage-insensitive gating after charge-neutralizing mutations in the S4 segment of Shaker channels.J Gen Physiol. 1999 Jan;113(1):139-51. doi: 10.1085/jgp.113.1.139. J Gen Physiol. 1999. PMID: 9874694 Free PMC article.
-
Gating of voltage-dependent potassium channels.Prog Biophys Mol Biol. 2001;75(3):165-99. doi: 10.1016/s0079-6107(01)00006-2. Prog Biophys Mol Biol. 2001. PMID: 11376798 Review.
-
Voltage sensors.Mol Pharmacol. 2025 Feb;107(2):100011. doi: 10.1016/j.molpha.2024.100011. Epub 2024 Dec 12. Mol Pharmacol. 2025. PMID: 40023511 Review.
Cited by
-
Coupling of voltage-sensors to the channel pore: a comparative view.Front Pharmacol. 2012 Jul 27;3:145. doi: 10.3389/fphar.2012.00145. eCollection 2012. Front Pharmacol. 2012. PMID: 22866036 Free PMC article.
-
Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the H(V) family.Physiol Rev. 2013 Apr;93(2):599-652. doi: 10.1152/physrev.00011.2012. Physiol Rev. 2013. PMID: 23589829 Free PMC article. Review.
-
An Unorthodox Mechanism Underlying Voltage Sensitivity of TRPV1 Ion Channel.Adv Sci (Weinh). 2020 Sep 21;7(20):2000575. doi: 10.1002/advs.202000575. eCollection 2020 Oct. Adv Sci (Weinh). 2020. PMID: 33101845 Free PMC article.
-
Down-state model of the voltage-sensing domain of a potassium channel.Biophys J. 2010 Jun 16;98(12):2857-66. doi: 10.1016/j.bpj.2010.03.031. Biophys J. 2010. PMID: 20550898 Free PMC article.
-
PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating.Front Physiol. 2014 May 27;5:195. doi: 10.3389/fphys.2014.00195. eCollection 2014. Front Physiol. 2014. PMID: 24904429 Free PMC article. Review.
References
-
- Aggarwal S., MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. - PubMed
-
- Almers W. Gating currents and charge movements in excitable membranes. Rev. Physiol. Biochem. Pharmacol. 1978;82:96–214. - PubMed
-
- Baker O.S., Larsson H.P., Mannuzzu L.M., Isacoff E.Y. Three transmembrane conformations and sequence-dependent displacement of the S4 domain in Shaker K+ channel gating. Neuron. 1998;20:1283–1294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

