Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells
- PMID: 10540157
- PMCID: PMC1905390
- DOI: 10.1046/j.1365-2249.1999.01016.x
Analysis of the secretion pattern of monocyte chemotactic protein-1 (MCP-1) and transforming growth factor-beta 2 (TGF-beta2) by human retinal pigment epithelial cells
Abstract
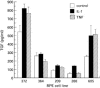
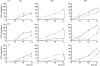
Retinal pigment epithelial (RPE) cells, situated between the neurosensory retina and the vascularized choroid, form part of the blood-eye barrier and are important for homeostasis of the outer retina. These cells are able to produce a variety of cytokines which may play a role in the maintenance of the immunosuppressive milieu inside the eye and in intraocular inflammatory responses. In the present study, we investigated whether RPE cells secreted the anti-inflammatory cytokine TGF-beta2 and the proinflammatory cytokine MCP-1 in a polarized manner. Monolayers of human donor RPE cells were cultured on transwell filters. Secretion of TGF-beta2 and MCP-1 at either the apical or basal side of the RPE cell monolayers, that were not treated or stimulated with IL-1beta (200 U/ml), was analysed by ELISA. All three cell lines examined had a different TGF-beta2 secretion pattern. In two of the three donor RPE cell lines tested, TGF-beta2 secretion was polarized, but not in the same direction. TGF-beta2 secretion was not up-regulated by stimulation with IL-1beta. In contrast, IL-1beta strongly induced MCP-1 secretion preferentially into the basal compartment of all RPE monolayers tested. These data indicate that human RPE cells are able to secrete TGF-beta2 and MCP-1 in a polarized fashion. Our results suggest that MCP-1 can be secreted by RPE cells in the direction of choroidal vessels during inflammatory responses in the posterior part of the eye, which may limit damage to the neurosensory retina.
Figures

Similar articles
-
Cell-associated human retinal pigment epithelium interleukin-8 and monocyte chemotactic protein-1: immunochemical and in-situ hybridization analyses.Exp Eye Res. 1997 Dec;65(6):781-9. doi: 10.1006/exer.1997.0380. Exp Eye Res. 1997. PMID: 9441701
-
Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells.Clin Exp Immunol. 1998 Apr;112(1):34-43. doi: 10.1046/j.1365-2249.1998.00560.x. Clin Exp Immunol. 1998. PMID: 9566787 Free PMC article.
-
Monocyte chemotactic protein gene expression by cytokine-treated human retinal pigment epithelial cells.Lab Invest. 1991 Jun;64(6):819-25. Lab Invest. 1991. PMID: 2046333
-
VIP enhances the differentiation of retinal pigment epithelium in culture: from cAMP and pp60(c-src) to melanogenesis and development of fluid transport capacity.Prog Retin Eye Res. 2000 Nov;19(6):669-88. doi: 10.1016/s1350-9462(00)00010-0. Prog Retin Eye Res. 2000. PMID: 11029551 Review.
-
Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes.Prog Retin Eye Res. 2001 Jan;20(1):29-48. doi: 10.1016/s1350-9462(00)00017-3. Prog Retin Eye Res. 2001. PMID: 11070367 Review.
Cited by
-
An in vitro model of the back of the eye for studying retinal pigment epithelial-choroidal endothelial interactions.In Vitro Cell Dev Biol Anim. 2002 Apr;38(4):228-34. doi: 10.1290/1071-2690(2002)038<0228:AIVMOT>2.0.CO;2. In Vitro Cell Dev Biol Anim. 2002. PMID: 12197775
-
Subretinal organization in stage 5 retinopathy of prematurity.Graefes Arch Clin Exp Ophthalmol. 2003 Apr;241(4):263-8. doi: 10.1007/s00417-003-0632-x. Epub 2003 Mar 18. Graefes Arch Clin Exp Ophthalmol. 2003. PMID: 12719986
-
Smoking and age-related macular degeneration: review and update.J Ophthalmol. 2013;2013:895147. doi: 10.1155/2013/895147. Epub 2013 Dec 4. J Ophthalmol. 2013. PMID: 24368940 Free PMC article. Review.
-
Cigarette smoke-related hydroquinone dysregulates MCP-1, VEGF and PEDF expression in retinal pigment epithelium in vitro and in vivo.PLoS One. 2011 Feb 28;6(2):e16722. doi: 10.1371/journal.pone.0016722. PLoS One. 2011. PMID: 21386905 Free PMC article.
-
Control of chemokine gradients by the retinal pigment epithelium.Invest Ophthalmol Vis Sci. 2008 Oct;49(10):4620-30. doi: 10.1167/iovs.08-1816. Epub 2008 Apr 30. Invest Ophthalmol Vis Sci. 2008. PMID: 18450597 Free PMC article.
References
-
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. - PubMed
-
- Tanihara H, Yoshida M, Matsumoto M, Yoshimura N. Identification of transforming growth factor-beta expressed in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1993;34:413–9. - PubMed
-
- Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH. Transforming growth factor beta 2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res. 1994;59:323–33. - PubMed
-
- Jaffe GJ, Van Le L, Valea F, et al. Expression of interleukin-1 alpha, interleukin-1 beta, and an interleukin-1 receptor antagonist in human retinal pigment epithelial cells. Exp Eye Res. 1992;55:325–35. - PubMed
-
- Holtkamp GM, de Vos AF, Kijlstra A, Peek R. Expression of multiple forms of IL-1 receptor antagonist (IL-1ra) by human retinal pigment epithelial cells: identification of a new IL-1ra exon. Eur J Immunol. 1999;29:215–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

