Induction of HIV-1-specific T cell responses by administration of cytokines in late-stage patients receiving highly active anti-retroviral therapy
- PMID: 10540163
- PMCID: PMC1905397
- DOI: 10.1046/j.1365-2249.1999.01012.x
Induction of HIV-1-specific T cell responses by administration of cytokines in late-stage patients receiving highly active anti-retroviral therapy
Abstract
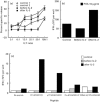
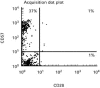
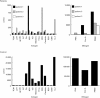
Highly active anti-retroviral therapy (HAART) is associated with reduction in the morbidity and mortality of patients with advanced HIV-1 disease. The ability of such treatment to improve immune responses against HIV-1 and opportunistic pathogens is variable and limited. Addition of cytokine immunotherapy to this treatment may improve immune responses. IL-2 with or without granulocyte-macrophage colony-stimulating factor (GM-CSF) was administered to HIV-1+ individuals receiving HAART with undetectable viral loads, and CD4 counts < 100 cells/microl. In one patient presenting with Mycobacterium avium complex (MAC) infection, we evaluated the effect of cytokine immunotherapy on lymphocyte phenotype; plasma viral load; proliferative responses to mitogens, recall and HIV-1 antigens; cytokine production and message in response to non-specific and specific stimuli; and natural killer (NK) cell activity. Proliferation assays were performed in two similar patients. Before cytokine immunotherapy the predominant CD8+ population was mainly CD28-. No proliferation or IL-2 production was seen in response to mitogens, recall or HIV-1 antigens; and no HIV-1 peptide-specific interferon-gamma (IFN-gamma)-secreting cells were present. Low levels of IL-4 were detected in response to antigens to which patients had been exposed, associated with up-regulated expression of costimulatory molecules influenced by IL-4. Following IL-2 administration, loss of IL-4 was associated with increased NK cell activity and HIV-1 peptide-specific and non-specific IFN-gamma-producing cells. Proliferative responses associated with IL-2 production and responsiveness were only seen after subsequent concomitant administration of GM-CSF with IL-2. These changes mirrored clinical improvement. An imbalance of lymphocyte subsets may account for immune unresponsiveness when receiving HAART. Restoration of responses following immunotherapy suggests a shift towards a lymphocyte profile with anti-pathogen activity.
Figures

Similar articles
-
Phase I trial of combined immunotherapy with subcutaneous granulocyte macrophage colony-stimulating factor, low-dose interleukin 2, and interferon alpha in progressive metastatic melanoma and renal cell carcinoma.Clin Cancer Res. 2000 Apr;6(4):1267-72. Clin Cancer Res. 2000. PMID: 10778950 Clinical Trial.
-
Unique cytokine production profile of anergic human T cells in SCID-hu mice after staphylococcal enterotoxin B administration.J Immunol. 1995 Apr 1;154(7):3204-12. J Immunol. 1995. PMID: 7534791
-
CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-gamma in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness.J Immunol. 2000 Aug 1;165(3):1685-91. doi: 10.4049/jimmunol.165.3.1685. J Immunol. 2000. PMID: 10903780
-
The challenge of IL-2 immunotherapy in HIV disease: "no through road" or turning point?Curr HIV Res. 2008 May;6(3):189-99. doi: 10.2174/157016208784325029. Curr HIV Res. 2008. PMID: 18473782 Review.
-
Defective phagocytosis by human monocyte/macrophages following HIV-1 infection: underlying mechanisms and modulation by adjunctive cytokine therapy.J Clin Virol. 2003 Feb;26(2):247-63. doi: 10.1016/s1386-6532(02)00123-3. J Clin Virol. 2003. PMID: 12600656 Review.
Cited by
-
Changes in T Cell and Dendritic Cell Phenotype from Mid to Late Pregnancy Are Indicative of a Shift from Immune Tolerance to Immune Activation.Front Immunol. 2017 Sep 15;8:1138. doi: 10.3389/fimmu.2017.01138. eCollection 2017. Front Immunol. 2017. PMID: 28966619 Free PMC article.
-
Immunological and virological consequences of patient-directed antiretroviral therapy interruption during chronic HIV-1 infection.Clin Exp Immunol. 2005 Nov;142(2):354-61. doi: 10.1111/j.1365-2249.2005.02918.x. Clin Exp Immunol. 2005. PMID: 16232224 Free PMC article.
-
Effects of recombinant human growth hormone on HIV-1-specific T-cell responses, thymic output and proviral DNA in patients on HAART: 48-week follow-up.J Immune Based Ther Vaccines. 2008 Oct 31;6:7. doi: 10.1186/1476-8518-6-7. J Immune Based Ther Vaccines. 2008. PMID: 18976455 Free PMC article.
-
Long-Term Non-Progression and Broad HIV-1-Specific Proliferative T-Cell Responses.Front Immunol. 2013 Mar 1;4:58. doi: 10.3389/fimmu.2013.00058. eCollection 2013. Front Immunol. 2013. PMID: 23459797 Free PMC article.
-
A balanced type 1/type 2 response is associated with long-term nonprogressive human immunodeficiency virus type 1 infection.J Virol. 2002 Sep;76(18):9011-23. doi: 10.1128/jvi.76.18.9011-9023.2002. J Virol. 2002. PMID: 12186885 Free PMC article.
References
-
- Gill J, Moyle G, Nelson M. Discontinuation of Mycobacterium avium complex prophylaxis in patients with a rise in CD4 count following highly active anti-retroviral therapy (HAART) AIDS. 1998;12:680. - PubMed
-
- Sepkowitz KA. Effect of HAART on natural history of AIDS-related opportunistic disorders. Lancet. 1998;351:228–30. - PubMed
-
- Race EM, Adelson-Mitty J, Kriegel GR, Barlam TF, Reimann KA, Letvin NL, Japour AJ. Focal mycobacterial lymphadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease. Lancet. 1998;351:252–5. - PubMed
-
- Carr A, Marriott D, Field A, Vasak E, Cooper DA. Treatment of HIV-1-associated microsporidiosis and cryptosporidiosis with combination antiretroviral therapy. Lancet. 1998;351:256–61. - PubMed
-
- Paxton WA, Martin SR, Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nature Med. 1996;2:412–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

