The activation of the neutrophil respiratory burst by anti-neutrophil cytoplasm autoantibody (ANCA) from patients with systemic vasculitis requires tyrosine kinases and protein kinase C activation
- PMID: 10540175
- PMCID: PMC1905400
- DOI: 10.1046/j.1365-2249.1999.01043.x
The activation of the neutrophil respiratory burst by anti-neutrophil cytoplasm autoantibody (ANCA) from patients with systemic vasculitis requires tyrosine kinases and protein kinase C activation
Abstract
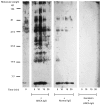
The ability of antineutrophil cytoplasm autoantibodies (ANCA) from patients with systemic vasculitis to stimulate protein kinase C (PKC) and tyrosine kinases was examined in human neutrophils. Using the superoxide dismutase-inhibitable reduction of ferricytochrome C, the kinetics of ANCA-induced superoxide (O2-) production were characterized and subsequently manipulated by specific inhibitors of PKC and tyrosine kinases. With this approach, ANCA IgG, but not normal IgG or ANCA F(ab')2 fragments caused a time and dose dependent release of O2- from TNF-alpha primed neutrophils. The kinetics of ANCA-induced O2- production showed an initial 10-15 min lag phase compared to the N-formyl-L-methionyl-L-leucyl-L-phenylalanine response, suggesting differences in the signalling pathways recruited by these two stimuli. Inhibitor studies revealed that ANCA-activation involved members of both the Ca2+-dependent and -independent PKC isoforms and also tyrosine kinases. ANCA IgG resulted in the translocation of the betaII isoform of PKC at a time corresponding to the end of the lag phase of O2- production, suggesting that PKC activity may be instrumental in processes regulating the activity of the NADPH oxidase in response to ANCA. Tyrosine phosphorylation of numerous proteins also peaked 10-15 min after stimulation with ANCA but not normal IgG. These data suggest that PKC and tyrosine kinases regulate O2- production from neutrophils stimulated with autoantibodies from patients with systemic vasculitis.
Figures





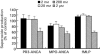
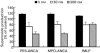
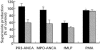

References
-
- Savage COS, Harper L, Adu D. Primary systemic vasculitis. Lancet. 1997;349:553–8. - PubMed
-
- Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–83. - PubMed
-
- Charles LA, Falk RJ, Jennette JC. Reactivity of ANCA with mononuclear phagocytes. J Leukocyte Biol. 1992;51:65–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

