Activation of human neutrophils by mycobacterial phenolic glycolipids
- PMID: 10540187
- PMCID: PMC1905428
- DOI: 10.1046/j.1365-2249.1999.01040.x
Activation of human neutrophils by mycobacterial phenolic glycolipids
Abstract
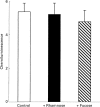
The interaction between mycobacterial phenolic glycolipids (PGLs) and phagocytes was studied. Human neutrophils were allowed to interact with each of four purified mycobacterial PGLs and the neutrophil production of reactive oxygen metabolites was followed kinetically by luminol-/isoluminol-amplified chemiluminescence. The PGLs from Mycobacterium tuberculosis and Mycobacterium kansasii, respectively, were shown to stimulate the production of oxygen metabolites, while PGLs from Mycobacterium marinum and Mycobacterium bovis BCG, respectively, were unable to induce an oxidative response. Periodate treatment of the M. tuberculosis PGL decreased the production of oxygen radicals, showing the importance of the PGL carbohydrate moiety for the interaction. The activation, however, could not be inhibited by rhamnose or fucose, indicating a complex interaction which probably involves more than one saccharide unit. This is in line with the fact that the activating PGLs from M. tuberculosis and M. kansasii contain tri- and tetrasaccharides, respectively, while the nonactivating PGLs from M. marinum and M. bovis BCG each contain a monosaccharide. The complement receptor 3 (CR3) has earlier been shown to be of importance for the phagocyte binding of mycobacteria, but did not appear to be involved in the activation of neutrophils by PGLs. The subcellular localization of the reactive oxygen metabolites formed was related to the way in which the glycolipids were presented to the cells.
Figures

 and M. marinum;
and M. marinum;  PGL) and 10 (M. tuberculosis; □ and M. kansasii; ▪ PGL) experiments. Ordinata, CL (arbitrary units).
PGL) and 10 (M. tuberculosis; □ and M. kansasii; ▪ PGL) experiments. Ordinata, CL (arbitrary units).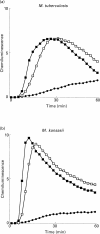
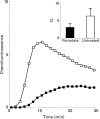
 ). The responses were measured in the presence of luminol and HRP. Ordinata, chemiluminescence (arbitrary units).
). The responses were measured in the presence of luminol and HRP. Ordinata, chemiluminescence (arbitrary units).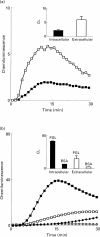

Similar articles
-
Specificity of Mycobacterium tuberculosis phenolic glycolipid (PGL-Tb1) antiserum.Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):535-45. doi: 10.1016/0769-2609(88)90153-6. Ann Inst Pasteur Microbiol. 1988. PMID: 2472828
-
Trisaccharides of Phenolic Glycolipids Confer Advantages to Pathogenic Mycobacteria through Manipulation of Host-Cell Pattern-Recognition Receptors.ACS Chem Biol. 2016 Oct 21;11(10):2865-2875. doi: 10.1021/acschembio.6b00568. Epub 2016 Sep 2. ACS Chem Biol. 2016. PMID: 27548027
-
Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii.J Infect Dis. 2002 Dec 15;186(12):1797-807. doi: 10.1086/345760. Epub 2002 Nov 19. J Infect Dis. 2002. PMID: 12447766
-
The carbohydrate- and lipid-containing cell wall of mycobacteria, phenolic glycolipids: structure and immunological properties.Crit Rev Microbiol. 1990;17(4):305-27. doi: 10.3109/10408419009105730. Crit Rev Microbiol. 1990. PMID: 2206395 Review.
-
Developments in the Synthesis of Mycobacterial Phenolic Glycolipids.Chem Rec. 2021 Nov;21(11):3295-3312. doi: 10.1002/tcr.202100200. Epub 2021 Sep 28. Chem Rec. 2021. PMID: 34581501 Review.
Cited by
-
Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice.Infect Immun. 2002 Sep;70(9):5322-7. doi: 10.1128/IAI.70.9.5322-5327.2002. Infect Immun. 2002. PMID: 12183593 Free PMC article.
-
The development of rapidly progressive glomerulonephritis associated with both antineutrophil cytoplasmic antibody-associated vasculitis and anti-glomerular basement membrane nephritis in the course of nontuberculous mycobacterium infection: a case report.BMC Rheumatol. 2020 Dec 14;4(1):68. doi: 10.1186/s41927-020-00167-y. BMC Rheumatol. 2020. PMID: 33308324 Free PMC article.
-
Antinuclear antibodies in Mycobacterium tuberculosis infection.Indian J Pediatr. 2008 Nov;75(11):1188. doi: 10.1007/s12098-008-0178-3. Indian J Pediatr. 2008. PMID: 18810350 No abstract available.
-
Human lung immunity against Mycobacterium tuberculosis: insights into pathogenesis and protection.Am J Respir Crit Care Med. 2011 Mar 15;183(6):696-707. doi: 10.1164/rccm.201006-0963PP. Epub 2010 Nov 12. Am J Respir Crit Care Med. 2011. PMID: 21075901 Free PMC article.
-
Autoantibody prevalence in active tuberculosis: reactive or pathognomonic?BMJ Open. 2013 Jul 26;3(7):e002665. doi: 10.1136/bmjopen-2013-002665. Print 2013. BMJ Open. 2013. PMID: 23892369 Free PMC article.
References
-
- Rook GAW, Bloom BR. Mechanism of pathogenesis in tuberculosis. In: Bloom BR, editor. Tuberculosis. Washington, DC: ASM Press; 1994. pp. 485–501.
-
- Rivière M, Fourniè JJ, Puzo G. A novel mannose containing phenolic glycolipid from Mycobacterium kansasii. J Biol Chem. 1987;262:14879–84. - PubMed
-
- Hartmann S, Minnikin DE. Mycobacterial phenolic glycolipids. In: Tyman JHP, editor. Surfactants in lipid chemistry. Cambridge: Royal Society of Chemistry; 1992. pp. 135–58.
-
- Daffé M, Lacave C, Laneelle MA, Laneelle G. Structure of the major triglycosyl phenol-phthiocerol of Mycobacterium tuberculosis(strain Canetti) Eur J Biochem. 1987;167:155–60. - PubMed
-
- Minnikin DE, Dobson G, Parlett JH, Goodfellow M, Magnusson M. Analysis of dimycocerosates of glycosyl phenolphthiocerols in the identification of some clinically significant mycobacteria. Eur J Clin Microbiol. 1987;6:703–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

