Induction of dendritic cell costimulator molecule expression is suppressed by T cells in the absence of antigen-specific signalling: role of cluster formation, CD40 and HLA-class II for dendritic cell activation
- PMID: 10540215
- PMCID: PMC2326915
- DOI: 10.1046/j.1365-2567.1999.00860.x
Induction of dendritic cell costimulator molecule expression is suppressed by T cells in the absence of antigen-specific signalling: role of cluster formation, CD40 and HLA-class II for dendritic cell activation
Abstract
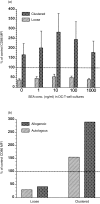
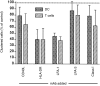
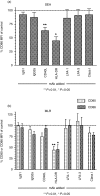
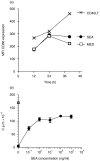
Full activation of T lymphocytes by dendritic cells (DC) during antigen presentation is known to require the interaction of several inducible receptor-ligand pairs. We have postulated that the reciprocal activation of DC by T lymphocytes is also important. Potential signalling molecules that might increase the stimulatory capacity of DC during antigen presentation to T lymphocytes were tested using an in vitro model. Fresh human blood DC were cocultured with CD4+ and CD8+ allogeneic or with autologous T lymphocytes plus Staphylococcus superantigen A (SEA). Surprisingly, costimulator expression on DC cocultured with T lymphocytes was reduced in comparison to DC cultured alone. However, the minority (10-30%) of DC clustering with T lymphocytes showed antigen-specific up-regulation of the CD40, CD80 and CD86 costimulator molecules, whereas the non-clustered DC (70-90%) had less up-regulation than control DC cultured alone and did not respond to antigen-specific triggering. Monoclonal antibodies (mAb) to CD40 ligand (CD40L) and human leucocyte antigen (HLA)-DR, but not lymphocyte function-associated antigen-1 (LFA-1), LFA-3 or HLA-class I, significantly inhibited the T-lymphocyte induction of DC costimulator expression. Since HLA-class II, but not HLA-class I mAb, inhibited allogeneic T-lymphocyte-mediated activation of DC, CD4 T lymphocytes appear to be the main subset activating DC in the mixed lymphocyte reaction. Cross-linking of CD40, but not HLA-class II, up-regulated DC or B-cell costimulator expression. Although direct class II signalling does not appear to play a role in DC activation, antigen-specific T-cell recognition contributes via other mechanisms to regulate DC activation.
Figures



Similar articles
-
Blockade of the CD40-CD40 ligand pathway potentiates the capacity of donor-derived dendritic cell progenitors to induce long-term cardiac allograft survival.Transplantation. 1997 Dec 27;64(12):1808-15. doi: 10.1097/00007890-199712270-00031. Transplantation. 1997. PMID: 9422424
-
Human dendritic cells activate T lymphocytes via a CD40: CD40 ligand-dependent pathway.Eur J Immunol. 1996 Jun;26(6):1204-10. doi: 10.1002/eji.1830260603. Eur J Immunol. 1996. PMID: 8647193
-
The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation.J Exp Med. 2004 Jun 21;199(12):1607-18. doi: 10.1084/jem.20040317. Epub 2004 Jun 14. J Exp Med. 2004. PMID: 15197224 Free PMC article.
-
The role of dendritic cells in T cell activation.Immunol Cell Biol. 1997 Jun;75(3):223-30. doi: 10.1038/icb.1997.35. Immunol Cell Biol. 1997. PMID: 9243286 Review.
-
CD40 and dendritic cell function.Crit Rev Immunol. 2003;23(1-2):83-107. doi: 10.1615/critrevimmunol.v23.i12.50. Crit Rev Immunol. 2003. PMID: 12906261 Review.
Cited by
-
Phenotypic characterization of five dendritic cell subsets in human tonsils.Am J Pathol. 2001 Jul;159(1):285-95. doi: 10.1016/S0002-9440(10)61694-X. Am J Pathol. 2001. PMID: 11438475 Free PMC article.
-
T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells.Nat Commun. 2018 Sep 7;9(1):3630. doi: 10.1038/s41467-018-06090-8. Nat Commun. 2018. PMID: 30194420 Free PMC article.
-
Spermidine suppresses DC activation via eIF5A hypusination and metabolic adaptation.Discov Immunol. 2025 May 15;4(1):kyaf009. doi: 10.1093/discim/kyaf009. eCollection 2025. Discov Immunol. 2025. PMID: 40510183 Free PMC article.
-
Dendritic cell density and activation status in human breast cancer -- CD1a, CMRF-44, CMRF-56 and CD-83 expression.Br J Cancer. 2002 Feb 12;86(4):546-51. doi: 10.1038/sj.bjc.6600132. Br J Cancer. 2002. PMID: 11870535 Free PMC article.
-
Tissue Damage Caused by Myeloablative, but Not Non-Myeloablative, Conditioning before Allogeneic Stem Cell Transplantation Results in Dermal Macrophage Recruitment without Active T-Cell Interaction.Front Immunol. 2018 Feb 27;9:331. doi: 10.3389/fimmu.2018.00331. eCollection 2018. Front Immunol. 2018. PMID: 29535719 Free PMC article. Clinical Trial.
References
-
- Hart DNJ. Dendritic cells: unique leucocyte populations which control the primary immune response. Blood. 1997;90:3245. - PubMed
-
- Hart DNJ, Calder VL. Human dendritic cells: function and cytokine production. Immunopharmacology of Macrophages and other Antigen-presenting Cells. In: Bruijnzeel-Loomen D, editor. Handbook of Immunopharmacology. London: Academic Press; 1994. p. 63.
-
- Peguet-Navarro J, Dalbiez-Gauthier C, Rattis F, van Kooten C, Banchereau J, Schmitt D. Functional expression of CD40 antigen on human epidermal Langerhans cells. J Immunol. 1995;155:4241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

