Lipopolysaccharide-dependent down-regulation of CD27 expression on T cells activated with superantigen
- PMID: 10540229
- PMCID: PMC2326921
- DOI: 10.1046/j.1365-2567.1999.00857.x
Lipopolysaccharide-dependent down-regulation of CD27 expression on T cells activated with superantigen
Abstract
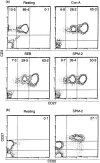
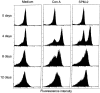
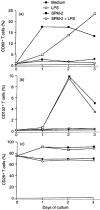
To investigate the mechanisms underlying T-cell responses during superantigen (SAg) stimulation, we analysed the effects of SAg on CD27 expression with or without lipopolysaccharide (LPS) as a novel regulator of T-cell function. CD27 is expressed on the majority of resting peripheral blood T cells (CD27low). Activation of T cells by SAg induces high levels of CD27 surface expression (CD27high) accompanied with simultaneous CD30 receptor expression. After prolonged activation in vitro, the level of CD27 expression became intermediate. The effects of LPS on down-regulation of CD27high expression on CD30+ T cells were dose-dependent. Separating LPS-stimulated monocytes from T cells by mechanical dispersion abolished its inhibitory effect, indicating the requirement for direct interactions between monocytes and T cells. We also found that SAg up-regulated CD80 expression on CD14+ monocytes and LPS inhibited SAg-induced CD80 expression after 24 hr of stimulation. Up-regulation of CD152 (CTLA-4) was selective, since it was found to be preferentially expressed on the CD30+ population. Competitive experiments using soluble blocking peptides showed that addition of CD28 or CD80 peptide recovered LPS-induced down-regulation of CD27high expression on CD30+ T cells. These observations suggested that the presence of low levels of CD80 on monocytes may partially inhibit CD27 expression due to inefficient delivery of positive signals via CD28/CD80 interaction, and that the increased levels of CD80 enhance the inhibition through interactions with CD152 which is expressed at the highest levels after 48 hr of activation.
Figures




Similar articles
-
B7-CD28 interaction is a late acting co-stimulatory signal for human T cell responses.Int Immunol. 1997 Aug;9(8):1095-102. doi: 10.1093/intimm/9.8.1095. Int Immunol. 1997. PMID: 9263006
-
Superantigen responses and co-stimulation: CD28 and CTLA-4 have opposing effects on T cell expansion in vitro and in vivo.Int Immunol. 1996 Apr;8(4):519-23. doi: 10.1093/intimm/8.4.519. Int Immunol. 1996. PMID: 8671638
-
Interaction of CTLA-4 (CD152) with CD80 or CD86 inhibits human T-cell activation.Immunology. 1999 Nov;98(3):413-21. doi: 10.1046/j.1365-2567.1999.00888.x. Immunology. 1999. PMID: 10583602 Free PMC article.
-
The Yin and Yang of T cell costimulation.Science. 1995 Nov 10;270(5238):932-3. doi: 10.1126/science.270.5238.932. Science. 1995. PMID: 7481795 Review. No abstract available.
-
CD28/CTLA-4 and CD80/CD86 families: signaling and function.Immunol Res. 1999;19(1):1-24. doi: 10.1007/BF02786473. Immunol Res. 1999. PMID: 10374692 Review.
Cited by
-
CD27-IgD- memory B cells are modulated by in vivo interleukin-6 receptor (IL-6R) blockade in rheumatoid arthritis.Arthritis Res Ther. 2015 Mar 14;17(1):61. doi: 10.1186/s13075-015-0580-y. Arthritis Res Ther. 2015. PMID: 25888920 Free PMC article.
-
Effects of superantigen and lipopolysaccharide on induction of CD80 through apoptosis of human monocytes.Infect Immun. 2001 Jun;69(6):3652-7. doi: 10.1128/IAI.69.6.3652-3657.2001. Infect Immun. 2001. PMID: 11349026 Free PMC article.
References
-
- Lee PK, Schlievert PM. Molecular genetics of pyrogenic exotoxin ‘superantigens’ of group A streptococci and Staphylococcus aureus. Curr Top Microbiol Immunol. 1991;174:21. - PubMed
-
- Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705. - PubMed
-
- Kotzin BL, Leung DYM, Kappler J, Marrack P. Superantigens and human disease. Adv Immunol. 1993;54:99. - PubMed
-
- Sato H, Itoh T, Rikiishi H, Kumagai K. Cytoplasmic membrane-associated protein (CAP) isolated from Streptococcus pyogenes: as a new bacterial superantigen. Microbiol Immunol. 1994;38:139. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

