Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF
- PMID: 10541554
- PMCID: PMC317101
- DOI: 10.1101/gad.13.20.2678
Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF
Abstract
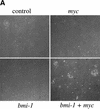
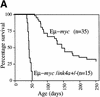
The bmi-1 and myc oncogenes collaborate strongly in murine lymphomagenesis, but the basis for this collaboration was not understood. We recently identified the ink4a-ARF tumor suppressor locus as a critical downstream target of the Polycomb-group transcriptional repressor Bmi-1. Others have shown that part of Myc's ability to induce apoptosis depends on induction of p19arf. Here we demonstrate that down-regulation of ink4a-ARF by Bmi-1 underlies its ability to cooperate with Myc in tumorigenesis. Heterozygosity for bmi-1 inhibits lymphomagenesis in Emu-myc mice by enhancing c-Myc-induced apoptosis. We observe increased apoptosis in bmi-1(-/-) lymphoid organs, which can be rescued by deletion of ink4a-ARF or overexpression of bcl2. Furthermore, Bmi-1 collaborates with Myc in enhancing proliferation and transformation of primary embryo fibroblasts (MEFs) in an ink4a-ARF dependent manner, by prohibiting Myc-mediated induction of p19arf and apoptosis. We observe strong collaboration between the Emu-myc transgene and heterozygosity for ink4a-ARF, which is accompanied by loss of the wild-type ink4a-ARF allele and formation of highly aggressive B-cell lymphomas. Together, these results reinforce the critical role of Bmi-1 as a dose-dependent regulator of ink4a-ARF, which on its turn acts to prevent tumorigenesis on activation of oncogenes such as c-myc.
Figures





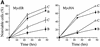
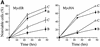
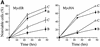












References
-
- Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman I. Bcl-2 rescues T lymphopoieses in Interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. - PubMed
-
- Alkema MJ, Jacobs H, van Lohuizen M, Berns A. Perturbation of B and T cell development and predisposition to lymphomagenesis in Eμ-Bmi1 transgenic mice require the Bmi1 RING finger. Oncogene. 1997;15:899–910. - PubMed
-
- Bissonette RP, Echeverri F, Mahboubi A, Green DR. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:522–554. - PubMed
-
- Blyth K, Terry A, O'Hara M, Baxter EW, Campbell M, Stewart M, Donehower LA, Onions DE, Neil JC, Cameron ER. Synergy between a human c-myc transgene and p53 null genotype in murine thymic lymphomas; contrasting effects of homozygous and heterozygous p53 loss. Oncogene. 1995;10:1717–1723. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
