Drug tolerance in Mycobacterium tuberculosis
- PMID: 10543735
- PMCID: PMC89531
- DOI: 10.1128/AAC.43.11.2600
Drug tolerance in Mycobacterium tuberculosis
Abstract
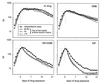
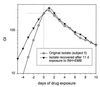
Although Mycobacterium tuberculosis is eradicated rapidly during therapy in some patients with pulmonary tuberculosis, it can persist for many months in others. This study examined the relationship between mycobacterial drug tolerance (delayed killing in vitro), persistence, and relapse. It was performed with 39 fully drug-susceptible isolates from a prospective trial of standard short-course antituberculous therapy with sputum smear-positive, human immunodeficiency virus-uninfected subjects with pulmonary tuberculosis in Brazil and Uganda. The rate of killing in vitro was determined by monitoring the growth index (GI) in BACTEC 12B medium after addition of drug to established cultures and was measured as the number of days required for 99% sterilization. Drugs differed significantly in bactericidal activity, in the following order from greatest to least, rifampin > isoniazid-ethambutol > ethambutol (P < 0.001). Isolates from subjects who had relapses (n = 2) or in whom persistence was prolonged (n = 1) were significantly more tolerant of isoniazid-ethambutol and rifampin than isolates from other subjects (P < 0.01). More generally, the duration of persistence during therapy was predicted by strain tolerance to isoniazid and rifampin (P = 0.012 and 0.026, respectively). Tolerance to isoniazid-ethambutol and tolerance to rifampin were highly correlated (P < 0.001). Tolerant isolates did not differ from others with respect to the MIC of isoniazid; the rate of killing of a tolerant isolate by isoniazid-ethambutol was not increased at higher drug concentrations. These observations suggest that tolerance may not be due to drug-specific mechanisms. Tolerance was of the phenotypic type, although increased tolerance appeared to emerge after prolonged drug exposure in vivo. This study suggests that drug tolerance may be an important determinant of the outcome of therapy for tuberculosis.
Figures
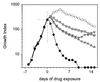
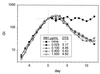
 , INH;
, INH;
 , INH-EMB; ●, RIF.
, INH-EMB; ●, RIF.

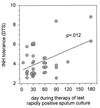


References
-
- Aber V R, Nunn A J. Short term chemotherapy of tuberculosis. Factors affecting relapse following short term chemotherapy. Bull Int Union Tuberc. 1978;53:276–280. - PubMed
-
- Anonymous. A controlled trial of 2-month, 3-month, and 12-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 60 months. Am Rev Respir Dis. 1984;130:23–28. - PubMed
-
- Anonymous. A controlled trial of 3-month, 4-month, and 6-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 5 years. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. Am Rev Respir Dis. 1989;139:871–876. - PubMed
-
- Balasubramanian R, Sivasubramanian S, Vijayan V K, Ramachandran R, Jawahar M S, Paramasivan C N, Selvakumar N, Somasundaram P R. Five year results of a 3-month and two 5-month regimens for the treatment of sputum-positive pulmonary tuberculosis in south India. Tubercle. 1990;71:253–258. - PubMed
-
- Bishai W R, Chaisson R E. Short-course chemoprophylaxis for tuberculosis. Clin Chest Med. 1997;18:115–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

