CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site
- PMID: 10544194
- PMCID: PMC2195676
- DOI: 10.1084/jem.190.9.1215
CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site
Abstract
Systemic tolerance can be elicited by introducing antigen into an immune-privileged site, such as the eye, or directly into the blood. Both routes of immunization result in a selective deficiency of systemic delayed type hypersensitivity. Although the experimental animal model of anterior chamber-associated immune deviation (ACAID) occurs in most mouse strains, ACAID cannot be induced in several mutant mouse strains that are coincidentally deficient in natural killer T (NKT) cells. Therefore, this model for immune-privileged site-mediated tolerance provided us with an excellent format for studying the role of NKT cells in the development of tolerance. The following data show that CD1-reactive NKT cells are required for the development of systemic tolerance induced via the eye as follows: (a) CD1 knockout mice were unable to develop ACAID unless they were reconstituted with NKT cells together with CD1(+) antigen-presenting cells; (b) specific antibody depletion of NKT cells in vivo abrogated the development of ACAID; and (c) anti-CD1 monoclonal antibody treatment of wild-type mice prevented ACAID development. Significantly, CD1-reactive NKT cells were not required for intravenously induced systemic tolerance, thereby establishing that different mechanisms mediate development of tolerance to antigens inoculated by these routes. A critical role for NKT cells in the development of systemic tolerance associated with an immune-privileged site suggests a mechanism involving NKT cells in self-tolerance and their defects in autoimmunity.
Figures
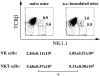



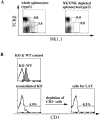

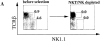
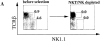




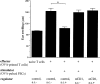
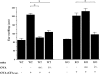
Comment in
-
Immune privilege: keeping an eye on natural killer T cells.J Exp Med. 1999 Nov 1;190(9):1197-200. doi: 10.1084/jem.190.9.1197. J Exp Med. 1999. PMID: 10544192 Free PMC article. No abstract available.
References
-
- Streilein J.W. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr. Opin. Immunol. 1993;5:428–432. - PubMed
-
- Griffith T.S., Brunner T., Fletcher S.M., Green D.R., Ferguson T.A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. - PubMed
-
- Taylor A.W., Streilein J.W., Cousins S.W. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr. Eye. Res. 1992;11:1199–1206. - PubMed
-
- Taylor A.W., Streilein J.W., Cousins S.W. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J. Immunol. 1994;153:1080–1086. - PubMed
-
- Streilein J.W., Bradley D. Analysis of immunosuppressive properties of iris and ciliary body cells and their secretory products. Invest. Ophthalmol. Vis. Sci. 1991;32:2700–2710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

