A critical role for CD40-CD40 ligand interactions in amplification of the mucosal CD8 T cell response
- PMID: 10544199
- PMCID: PMC2195681
- DOI: 10.1084/jem.190.9.1275
A critical role for CD40-CD40 ligand interactions in amplification of the mucosal CD8 T cell response
Abstract
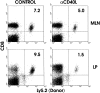
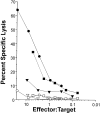
The role of CD40 ligand (CD40L) in CD8 T cell activation was assessed by tracking antigen-specific T cells in vivo using both adoptive transfer of T cell receptor transgenic T cells and major histocompatibility complex (MHC) class I tetramers. Soluble antigen immunization induced entry of CD8 cells into the intestinal mucosa and cytotoxic T lymphocyte (CTL) differentiation, whereas CD8 cells in secondary lymphoid tissue proliferated but were not cytolytic. Immunization concurrent with CD40L blockade or in the absence of CD40 demonstrated that accumulation of CD8 T cells in the mucosa was CD40L dependent. Furthermore, activation was mediated through CD40L expressed by the CD8 cells, since inhibition by anti-CD40L monoclonal antibodies occurred after adoptive transfer to CD40L-deficient mice. However, mucosal CD8 T cells in normal and CD40(-/-) mice were equivalent killers, indicating that CD40L was not required for CTL differentiation. Appearance of virus-specific mucosal, but not splenic, CD8 cells also relied heavily on CD40-CD40L interactions. The mucosal CTL response of transferred CD8 T cells was MHC class II and interleukin 12 independent. The results established a novel pathway of direct CD40L-mediated CD8 T cell activation.
Figures






Similar articles
-
Soluble antigen and CD40 triggering are sufficient to induce primary and memory cytotoxic T cells.J Immunol. 2000 Jan 15;164(2):725-32. doi: 10.4049/jimmunol.164.2.725. J Immunol. 2000. PMID: 10623816
-
CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T cell functions.J Exp Med. 1996 May 1;183(5):2209-18. doi: 10.1084/jem.183.5.2209. J Exp Med. 1996. PMID: 8642330 Free PMC article.
-
CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response.J Exp Med. 1996 May 1;183(5):2129-42. doi: 10.1084/jem.183.5.2129. J Exp Med. 1996. PMID: 8642323 Free PMC article.
-
The role of CD40 ligand in costimulation and T-cell activation.Immunol Rev. 1996 Oct;153:85-106. doi: 10.1111/j.1600-065x.1996.tb00921.x. Immunol Rev. 1996. PMID: 9010720 Review.
-
The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity.J Leukoc Biol. 2000 May;67(5):607-14. doi: 10.1002/jlb.67.5.607. J Leukoc Biol. 2000. PMID: 10810999 Review.
Cited by
-
Dendritic cell maturation enhances CD8+ T-cell responses to exogenous antigen via a proteasome-independent mechanism of major histocompatibility complex class I loading.Immunology. 2003 Jul;109(3):374-83. doi: 10.1046/j.1365-2567.2003.01664.x. Immunology. 2003. PMID: 12807483 Free PMC article.
-
Reflections on CD8 T-cell activation and memory.Immunol Res. 2004;29(1-3):151-60. doi: 10.1385/IR:29:1-3:151. Immunol Res. 2004. PMID: 15181278 Review.
-
Induction of B-cell immune tolerance by antigen-modified cytotoxic T lymphocytes.Transplantation. 2010 Mar 27;89(6):667-76. doi: 10.1097/TP.0b013e3181ca9048. Transplantation. 2010. PMID: 20065917 Free PMC article.
-
Initial T cell frequency dictates memory CD8+ T cell lineage commitment.Nat Immunol. 2005 Aug;6(8):793-9. doi: 10.1038/ni1227. Epub 2005 Jul 17. Nat Immunol. 2005. PMID: 16025119 Free PMC article.
-
What's Sex Got to Do With COVID-19? Gender-Based Differences in the Host Immune Response to Coronaviruses.Front Immunol. 2020 Aug 28;11:2147. doi: 10.3389/fimmu.2020.02147. eCollection 2020. Front Immunol. 2020. PMID: 32983176 Free PMC article. Review.
References
-
- Janeway C.A., Jr., Bottomly K. Signals and signs for lymphocyte responses. Cell. 1994;76:275–285. - PubMed
-
- Germain R.N. MHC-dependent antigen processing and peptide presentationproviding ligands for T lymphocyte activation. Cell. 1994;76:287–299. - PubMed
-
- Kearney E.R., Pape K.A., Loh D.Y., Jenkins M.K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo . Immunity. 1994;1:327–339. - PubMed
-
- Pape K.A., Kearney E.R., Khoruts A., Mondino A., Merica R., Chen Z.M., Ingulli E., White J., Johnson J.G., Jenkins M.K. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cells for the study of T-cell activation in vivo. Immunol. Rev. 1997;156:67–78. - PubMed
-
- Lenschow D.J., Walunas T.L., Bluestone J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996;14:233–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

