Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes
- PMID: 10544204
- PMCID: PMC2195687
- DOI: 10.1084/jem.190.9.1329
Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes
Abstract
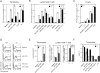
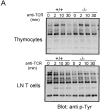
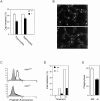
The Wiskott-Aldrich syndrome protein (WASp) has been implicated in modulation of lymphocyte activation and cytoskeletal reorganization. To address the mechanisms whereby WASp subserves such functions, we have examined WASp roles in lymphocyte development and activation using mice carrying a WAS null allele (WAS(-)(/)(-)). Enumeration of hemopoietic cells in these animals revealed total numbers of thymocytes, peripheral B and T lymphocytes, and platelets to be significantly diminished relative to wild-type mice. In the thymus, this abnormality was associated with impaired progression from the CD44(-)CD25(+) to the CD44(-)CD25(-) stage of differentiation. WASp-deficient thymocytes and T cells also exhibited impaired proliferation and interleukin (IL)-2 production in response to T cell antigen receptor (TCR) stimulation, but proliferated normally in response to phorbol ester/ionomycin. This defect in TCR signaling was associated with a reduction in TCR-evoked upregulation of the early activation marker CD69 and in TCR-triggered apoptosis. While induction of TCR-zeta, ZAP70, and total protein tyrosine phosphorylation as well as mitogen-activated protein kinase (MAPK) and stress-activated protein/c-Jun NH(2)-terminal kinase (SAPK/JNK) activation appeared normal in TCR-stimulated WAS(-)(/)(-) cells, TCR-evoked increases in intracellular calcium concentration were decreased in WASp-deficient relative to wild-type cells. WAS(-)(/)(-) lymphocytes also manifested a marked reduction in actin polymerization and both antigen receptor capping and endocytosis after TCR stimulation, whereas WAS(-)(/)(-) neutrophils exhibited reduced phagocytic activity. Together, these results provide evidence of roles for WASp in driving lymphocyte development, as well as in the translation of antigen receptor stimulation to proliferative or apoptotic responses, cytokine production, and cytoskeletal rearrangement. The data also reveal a role for WASp in modulating endocytosis and phagocytosis and, accordingly, suggest that the immune deficit conferred by WASp deficiency reflects the disruption of a broad range of cellular behaviors.
Figures







References
-
- Aldrich R.A., Steinberg A.G., Campbell D.C. Pedigree demonstrating a sex-linked recessive condition characterized by draining ears, eczematoid dermatitis and bloody diarrhea. Paediatrics. 1954;13:133–138. - PubMed
-
- Cooper M.D., Chase H.P., Lowman J.T., Krivit W., Good R.A. The Wiskott-Aldrich syndromean immunologic deficiency disease involving the afferent limb of immunity. Am. J. Med. 1968;44:499–513. - PubMed
-
- Ochs H.D., Slichter S.J., Harker L.A., von Behrens W.E., Clark R.A., Wedgwood R.J. The Wiskott-Aldrich syndromestudies of lymphocytes, granulocytes, and platelets. Blood. 1980;55:243–252. - PubMed
-
- Spitler L.E., Levin A.S., Stites D.P., Fudenberg H.H., Huber H.H. The Wiskott-Aldrich syndrome. Immunologic studies in nine patients and selected family members. Cell. Immunol. 1975;19:201–218. - PubMed
-
- Grottum K.A., Hovig T., Holmsen H., Abrahamson A.F., Jeremic M., Seip M. Wiskott-Aldrich syndromequalitative platelet defects and short platelet survival. Br. J. Haematol. 1969;17:373–388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

