Action of internal pronase on the f-channel kinetics in the rabbit SA node
- PMID: 10545140
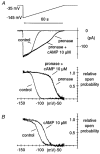
- PMCID: PMC2269621
- DOI: 10.1111/j.1469-7793.1999.00737.x
Action of internal pronase on the f-channel kinetics in the rabbit SA node
Abstract
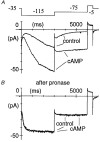
1. The hyperpolarization-activated If current was recorded in inside-out macropatches from sino-atrial (SA) node myocytes during exposure of their intracellular side to pronase, in an attempt to verify if cytoplasmic f-channel domains are involved in both voltage- and cAMP-dependent gating. 2. Superfusion with pronase caused a quick, dramatic acceleration of channel opening upon hyperpolarization and slowing, rapidly progressing into full blockade, of channel closing upon depolarization; these changes persisted after wash off of pronase and were irreversible, indicating proteolytic cleavage of channel regions which contribute to gating. 3. If recorded from patches normally responding to cAMP became totally insensitive to cAMP following pronase treatment, indicating partial or total removal of channel regions involved in the cAMP-dependent activation. 4. The fully activated I-V relationship was not modified by pronase, indicating that internal proteolysis did not affect the f-channel conductance. 5. The changes in If kinetics induced by pronase were due to a large depolarizing shift of the f-channel open probability curve (56.5 +/- 1.1 mV, n = 7). 6. These results are consistent with the hypothesis that cytoplasmic f-channel regions are implicated in dual voltage- and cAMP-dependent gating; also, since pronase does not abolish hyperpolarization-activated opening, an intrinsic voltage-dependent gating mechanism must exist which is inaccessible to proteolytic cleavage. A model scheme able to account for these data thus includes an intrinsic gating mechanism operating at depolarized voltages, and a blocking mechanism coupled to cAMP binding to the channel.
Figures


References
-
- Accili EA, DiFrancesco D. Inhibition of the hyperpolarization-activated current (if) of rabbit SA node myocytes by niflumic acid. Pflügers Archiv. 1996;431:757–762. - PubMed
-
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
-
- Clapham D. Not so funny anymore: pacing channels are cloned. Neuron. 1998;21:5–7. - PubMed
-
- Covarrubias M, Wei A, Salkoff L. Shaker, Shal, Shab and Shaw express independent K+ current systems. Neuron. 1991;7:763–773. - PubMed
-
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annual Reviews of Physiology. 1993;55:451–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

