Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival
- PMID: 10545499
- PMCID: PMC2151183
- DOI: 10.1083/jcb.147.3.545
Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival
Abstract
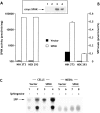
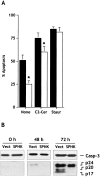
Sphingosine-1-phosphate (SPP) is a bioactive lipid that has recently been identified as the ligand for the EDG family of G protein-coupled cell surface receptors. However, the mitogenic and survival effects of exogenous SPP may not correlate with binding to cell-surface receptors (Van Brocklyn, J.R., M.J. Lee, R. Menzeleev, A. Olivera, L. Edsall, O. Cuvillier, D.M. Thomas, P.J.P. Coopman, S. Thangada, T. Hla, and S. Spiegel. 1998. J. Cell Biol. 142:229-240). The recent cloning of sphingosine kinase, a unique lipid kinase responsible for the formation of SPP, has provided a new tool to investigate the role of intracellular SPP. Expression of sphingosine kinase markedly increased SPP levels in NIH 3T3 fibroblasts and HEK293 cells, but no detectable secretion of SPP into the medium was observed. The increased sphingosine kinase activity in NIH 3T3 fibroblasts was sufficient to promote growth in low- serum media, expedite the G(1)/S transition, and increase DNA synthesis and the proportion of cells in the S phase of the cell cycle with a concomitant increase in cell numbers. Transient or stable overexpression of sphingosine kinase in NIH 3T3 fibroblasts or HEK293 cells protected against apoptosis induced by serum deprivation or ceramide elevation. N,N-Dimethylsphingosine, a competitive inhibitor of sphingosine kinase, blocked the effects of sphingosine kinase overexpression on cell proliferation and suppression of apoptosis. In contrast, pertussis toxin did not abrogate these biological responses. In Jurkat T cells, overexpression of sphingosine kinase also suppressed serum deprivation- and ceramide-induced apoptosis and, to a lesser extent, Fas-induced apoptosis, which correlated with inhibition of DEVDase activity, as well as inhibition of the executionary caspase-3. Taken together with ample evidence showing that growth and survival factors activate sphingosine kinase, our results indicate that SPP functions as a second messenger important for growth and survival of cells. Hence, SPP belongs to a novel class of lipid mediators that can function inside and outside cells.
Figures





References
-
- An S., Bleu T., Huang W., Hallmark O.G., Coughling S.R., Goetzl E.J. Identification of cDNAs encoding two G protein–coupled receptors for lysosphingolipids. FEBS Lett. 1997;417:279–282. - PubMed
-
- Bornfeldt K.E., Graves L.M., Raines E.W., Igarashi Y., Wayman G., Yamamura S., Yatomi Y., Sidhu J.S., Krebs E.G., Hakomori S., Ross R. Sphingosine-1-phosphate inhibits PDGF-induced chemotaxis of human arterial smooth muscle cellsspatial and temporal modulation of PDGF chemotactic signal transduction. J. Cell Biol. 1995;130:193–206. - PMC - PubMed
-
- Buehrer B.M., Bardes E.S., Bell R.M. Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim. Biophys. Acta. 1996;1303:233–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

