PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis
- PMID: 10545500
- PMCID: PMC2151188
- DOI: 10.1083/jcb.147.3.559
PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis
Abstract
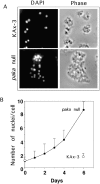
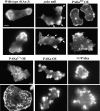
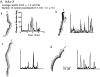
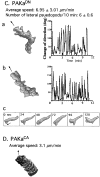
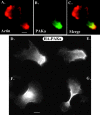
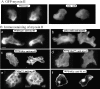
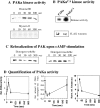
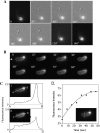
We have identified a Dictyostelium discoideum gene encoding a serine/threonine kinase, PAKa, a putative member of the Ste20/PAK family of p21-activated kinases, with a kinase domain and a long NH(2)-terminal regulatory domain containing an acidic segment, a polyproline domain, and a CRIB domain. PAKa colocalizes with myosin II to the cleavage furrow of dividing cells and the posterior of polarized, chemotaxing cells via its NH(2)-terminal domain. paka null cells are defective in completing cytokinesis in suspension. PAKa is also required for maintaining the direction of cell movement, suppressing lateral pseudopod extension, and proper retraction of the posterior of chemotaxing cells. paka null cells are defective in myosin II assembly, as the myosin II cap in the posterior of chemotaxing cells and myosin II assembly into cytoskeleton upon cAMP stimulation are absent in these cells, while constitutively active PAKa leads to an upregulation of myosin II assembly. PAKa kinase activity against histone 2B is transiently stimulated and PAKa incorporates into the cytoskeleton with kinetics similar to those of myosin II assembly in response to chemoattractant signaling. However, PAKa does not phosphorylate myosin II. We suggest that PAKa is a major regulator of myosin II assembly, but does so by negatively regulating myosin II heavy chain kinase.
Figures





References
-
- Abu-Elneel K., Karchi M., Ravid S. Dictyostelium myosin II is regulated during chemotaxis by a novel protein kinase C. J. Biol. Chem. 1996;271:977–984. - PubMed
-
- Bagrodia S., Dérijard B., Davis R.J., Cerione R.A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J. Biol. Chem. 1995;270:27995–27998. - PubMed
-
- Berlot C.H., Spudich J.A., Devreotes P.N. Chemoattractant-elicited increases in myosin phosphorylation in Dictyostelium . Cell. 1985;43:307–314. - PubMed
-
- Bokoch G.M., Wang Y., Bohl B.P., Sells M.A., Quilliam L.A., Knaus U.G. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J. Biol. Chem. 1996;271:25746–25749. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

