Comparative analysis of two meningococcal immunotyping monoclonal antibodies by resonant mirror biosensor and antibody gene sequencing
- PMID: 10548573
- PMCID: PMC95785
- DOI: 10.1128/CDLI.6.6.838-843.1999
Comparative analysis of two meningococcal immunotyping monoclonal antibodies by resonant mirror biosensor and antibody gene sequencing
Abstract
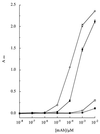
Lipooligosaccharide (LOS) is a major surface component of the cell walls of Neisseria meningitidis, which is important for its roles in pathogenesis and antigenic variation, as a target for immunological typing, and as a possible vaccine component. Although the structures of many antigenic variants have been determined, routine immunological typing of these molecules remains problematic. Resonant mirror analysis was combined with gene sequencing to characterize two monoclonal antibodies (MAbs) used in typing panels that were raised against the same LOS immunotype, L3,7,9. The two MAbs (MAb 4A8-B2 and MAb 9-2-L379) were of the same immunoglobulin subtype, but while MAb 9-2-L379 was more than a 1,000-fold more sensitive in immunotyping assays of both whole meningococcal cells and purified LOS, MAb 4A8-B2 was more specific for immunotype L3,7,9. The differences in sensitivity were a consequence of MAb 9-2-L379 having a 44-fold-faster association constant than MAb 4A8-B2. Comparison of the amino acid sequences of the variable chains of the MAbs revealed that they had very similar heavy chains (81% amino acid sequence identity) but diverse light chains (54% sequence identity). The differential binding kinetics and specificities observed with these MAbs were probably due to differences in the epitopes recognized, and these were probably a consequence of the different immunization protocols used in their production.
Figures



Similar articles
-
Development, characterization, and biological properties of meningococcal immunotype L3,7,(8),9-specific monoclonal antibodies.Clin Diagn Lab Immunol. 1994 Nov;1(6):729-36. doi: 10.1128/cdli.1.6.729-736.1994. Clin Diagn Lab Immunol. 1994. PMID: 8556528 Free PMC article.
-
Functional activities and immunoglobulin variable regions of human and murine monoclonal antibodies specific for the P1.7 PorA protein loop of Neisseria meningitidis.Infect Immun. 2000 Apr;68(4):1871-8. doi: 10.1128/IAI.68.4.1871-1878.2000. Infect Immun. 2000. PMID: 10722576 Free PMC article.
-
Protection against infection with Neisseria meningitidis group B serotype 2b by passive immunization with serotype-specific monoclonal antibody.Infect Immun. 1985 Nov;50(2):510-6. doi: 10.1128/iai.50.2.510-516.1985. Infect Immun. 1985. PMID: 3932211 Free PMC article.
-
Murine immune response to Neisseria meningitidis group C capsular polysaccharide: analysis of monoclonal antibodies generated in response to a thymus-independent antigen and a thymus-dependent toxoid conjugate vaccine.Infect Immun. 2000 Jan;68(1):239-46. doi: 10.1128/IAI.68.1.239-246.2000. Infect Immun. 2000. PMID: 10603394 Free PMC article.
-
Molecular mimicry of host structures by lipooligosaccharides of Neisseria meningitidis: characterization of sialylated and nonsialylated lacto-N-neotetraose (Galbeta1-4GlcNAcbeta1-3Galbeta1-4Glc) structures in lipooligosaccharides using monoclonal antibodies and specific lectins.Adv Exp Med Biol. 2001;491:525-42. doi: 10.1007/978-1-4615-1267-7_35. Adv Exp Med Biol. 2001. PMID: 14533820 Review.
References
-
- Abdillahi H, Poolman J T. Whole-cell ELISA for typing Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol Lett. 1987;48:367–371. - PubMed
-
- Alfthan K. Surface plasmon resonance biosensors as a tool in antibody engineering. Biosens Bioelectron. 1998;13:653–663. - PubMed
-
- Cartwright K A V. Meningococcal disease. Chichester, England: Wiley; 1995.
-
- Davies R J, Pollard Knight D. An optical biosensor system for molecular interaction studies. Am Biotechnol Lab. 1993;11:52–54. - PubMed
-
- Diaz-Romero J, Outschoorn I. Selective biotinylation of Neisseria meningitidis group B capsular polysaccharide and application in an improved ELISA for the detection of specific antibodies. J Immunol Methods. 1993;160:35–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

