Use of monoclonal antibody in diagnosis of candidiasis caused by Candida albicans: detection of circulating aspartyl proteinase antigen
- PMID: 10548587
- PMCID: PMC95799
- DOI: 10.1128/CDLI.6.6.924-929.1999
Use of monoclonal antibody in diagnosis of candidiasis caused by Candida albicans: detection of circulating aspartyl proteinase antigen
Abstract
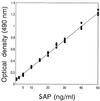
To develop a serological diagnosis of invasive candidiasis based on detection of circulating secreted aspartyl proteinase (SAP) antigen of Candida albicans, three different enzyme-linked immunosorbent assays (ELISAs) were compared. The first was a standard ELISA to detect anti-SAP antibodies, and the others were an antigen capture ELISA and an inhibition ELISA to detect circulating SAP antigen with monoclonal antibody (MAb) CAP1, which is highly specific for SAP. These tests were applied to 33 serum samples retrospectively selected from 33 patients with mycologically and/or serologically proven invasive candidiasis caused by C. albicans. Serum samples from 12 patients with aspergillosis and serum samples from 13 healthy individuals were also included. The sensitivities and specificities were 69.7 and 76.0% for the standard ELISA and 93.9 and 92.0% for the antigen capture ELISA, respectively. However, these values reached 93.9 and 96.0%, respectively, for the inhibition ELISA. Serum samples from 31 of 33 patients had detectable SAP antigen, with concentrations ranging from 6.3 to 19.0 ng/ml. These results indicate that the inhibition ELISA with MAb CAP1 is effective in detection of circulating SAP antigen and that this assay may be useful for diagnosis and treatment monitoring of invasive candidiasis.
Figures

Similar articles
-
Competitive binding inhibition enzyme-linked immunosorbent assay that uses the secreted aspartyl proteinase of Candida albicans as an antigenic marker for diagnosis of disseminated candidiasis.Clin Diagn Lab Immunol. 2003 Sep;10(5):835-48. doi: 10.1128/cdli.10.5.835-848.2003. Clin Diagn Lab Immunol. 2003. PMID: 12965914 Free PMC article.
-
Production of monoclonal antibodies for detection of a secreted aspartyl proteinase from Candida spp. in biologic specimens.Hybridoma (Larchmt). 2007 Aug;26(4):201-10. doi: 10.1089/hyb.2007.007. Hybridoma (Larchmt). 2007. PMID: 17725381
-
Production of hybrid phage displaying secreted aspartyl proteinase epitope of Candida albicans and its application for the diagnosis of disseminated candidiasis.Mycoses. 2007 May;50(3):165-71. doi: 10.1111/j.1439-0507.2006.01349.x. Mycoses. 2007. PMID: 17472610
-
Protective antigens and mechanisms of anti-Candida immunity.Nihon Ishinkin Gakkai Zasshi. 2000;41(4):219. doi: 10.3314/jjmm.41.219. Nihon Ishinkin Gakkai Zasshi. 2000. PMID: 11064318 Review.
-
Candida albicans secreted aspartyl proteinases.Curr Top Med Mycol. 1996 Dec;7(1):55-69. Curr Top Med Mycol. 1996. PMID: 9504059 Review.
Cited by
-
Distribution of secreted aspartyl proteinases using a polymerase chain reaction assay with SAP specific primers in Candida albicans isolates.Folia Microbiol (Praha). 2005;50(5):409-13. doi: 10.1007/BF02931422. Folia Microbiol (Praha). 2005. PMID: 16475500
-
New approach for diagnosis of candidemia based on detection of a 65-kilodalton antigen.Clin Vaccine Immunol. 2009 Nov;16(11):1538-45. doi: 10.1128/CVI.00176-09. Epub 2009 Sep 23. Clin Vaccine Immunol. 2009. PMID: 19776195 Free PMC article.
-
Secreted Aspartic Proteinases: Key Factors in Candida Infections and Host-Pathogen Interactions.Int J Mol Sci. 2024 Apr 27;25(9):4775. doi: 10.3390/ijms25094775. Int J Mol Sci. 2024. PMID: 38731993 Free PMC article. Review.
-
Competitive binding inhibition enzyme-linked immunosorbent assay that uses the secreted aspartyl proteinase of Candida albicans as an antigenic marker for diagnosis of disseminated candidiasis.Clin Diagn Lab Immunol. 2003 Sep;10(5):835-48. doi: 10.1128/cdli.10.5.835-848.2003. Clin Diagn Lab Immunol. 2003. PMID: 12965914 Free PMC article.
-
Expression and Purification along with Evaluation of Serological Response and Diagnostic Potential of Recombinant Sap2 Protein from C. parapsilosis for Use in Systemic Candidiasis.J Fungi (Basel). 2021 Nov 23;7(12):999. doi: 10.3390/jof7120999. J Fungi (Basel). 2021. PMID: 34946982 Free PMC article.
References
-
- Bodey G P, Fainstein V. Candidiasis. In: Bodey G P, Fainstein V, editors. Candidiasis. New York, N.Y: Raven Press; 1985. pp. 53–54.
-
- Bodey G P, Anaissie E J. Chronic systemic candidiasis. Eur J Clin Microbiol Infect Dis. 1989;8:855–857. - PubMed
-
- Buckley H R, Richardson M D, Evans E G V, Wheat L J. Immunodiagnosis of invasive fungal infection. J Med Vet Mycol. 1992;30(Suppl. 1):249–260. - PubMed
-
- Chakrabarti A, Roy P, Kumar D, Sharma B K, Chugh K S, Panigrahi D. Evaluation of three serological tests for detection of anti-candidal antibodies in diagnosis of invasive candidiasis. Mycopathologia. 1994;126:3–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

