Hepatocarcinoma-intestine-pancreas/pancreatic associated protein (HIP/PAP) is expressed and secreted by proliferating ductules as well as by hepatocarcinoma and cholangiocarcinoma cells
- PMID: 10550309
- PMCID: PMC1866987
- DOI: 10.1016/S0002-9440(10)65468-5
Hepatocarcinoma-intestine-pancreas/pancreatic associated protein (HIP/PAP) is expressed and secreted by proliferating ductules as well as by hepatocarcinoma and cholangiocarcinoma cells
Abstract
Hepatocarcinoma-intestine-pancreas/pancreatic associated protein (HIP/PAP) gene was identified because of its increased expression in 25% of human hepatocellular carcinoma. HIP/PAP protein, a C-type lectin, binds laminin, acts as an adhesion molecule for hepatocytes, and has also been described as an acute phase secretory protein during acute pancreatitis in humans and rats. We investigated HIP/PAP protein expression in patients with various liver diseases associated with ductular reaction. At the same time, we analyzed patients with hepatocellular carcinoma and cholangiocarcinoma, and tested HIP/PAP protein levels in sera to establish the pattern of secretion. Our data show that HIP/PAP expression was not restricted to hepatocellular carcinoma, but was also detected in cholangiocarcinoma cells as well as in reactive non-malignant bile ductules. In contrast, HIP/PAP protein expression was undetectable in normal mature hepatocytes, but some ductular cells localized at the interface of portal tracts with parenchyma were HIP/PAP immunoreactive in normal liver. Finally, we present evidence that HIP/PAP serum levels were increased in 21/28 (75%) patients with hepatocellular carcinoma, and in 25/51 (49%) patients with nonmalignant cirrhosis. Altogether, these results suggest that HIP/PAP protein may be implicated in hepatocytic and cholangiolar differentiation and proliferation.
Figures


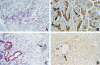

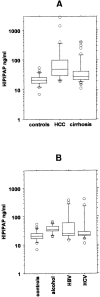
References
-
- Lasserre C, Christa L, Simon MT, Vernier P, Bréchot C: A novel gene (HIP) activated in human liver cancer. Cancer Res 1992, 52:5089-5095 - PubMed
-
- Drickamer K: Calcium-dependent carbohydrate-recognition domains in animal proteins. Curr Opin Struct Biol 1993, 3:393-400
-
- Frigerio JM, Dusetti NJ, Garrido P, Dagorn JC, Iovanna JL: The pancreatitis associated protein III (PAPIII), a new member of the PAP gene family. Biophys Biophys Acta 1993, 1216:329-331 - PubMed
-
- Narushima Y, Unno M, Nakagawa KI, Mori M, Miyashita H, Suzuki Y, Nogushi N, Takasawa S, Kumagai T, Yonekura H, Okamoto H: Structure, chromosomal localization, and expression of mouse genes encoding type III Reg, Reg IIIa, Reg IIIb, Reg IIIc. Gene 1997, 185:159-168 - PubMed
-
- Itoh T, Teraoka H: Cloning and tissue-specific expression of cDNAs for the human and mouse homologues of rat pancreatitis-associated protein (PAP). Biophys Biophys Acta 1993, 1172:184-186 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

