Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin
- PMID: 10550315
- PMCID: PMC1866989
- DOI: 10.1016/S0002-9440(10)65474-0
Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin
Abstract
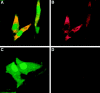
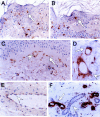
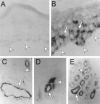
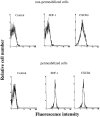
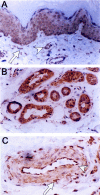
Stromal-cell derived factor or SDF-1 is a CXC chemokine constitutively expressed by stromal bone marrow cell cultures that binds to the G-protein-coupled receptor CXCR4. SDF-1/CXCR4 represents a unique, nonpromiscuous ligand/receptor pair that plays an essential role in prenatal myelo- and lymphopoiesis as well as in cardiovascular and neural development. SDF-1 prevents entry of CXCR4-dependent (X4) HIV viruses in T lymphocytes, by binding and internalizing CXCR4. The expression pattern of SDF-1 protein in normal tissues is not known. Here we describe an analysis of SDF-1 mRNA and protein in normal and inflamed skin by in situ hybridization and immunohistochemistry, using a novel anti-SDF-1 monoclonal antibody. We also describe the expression pattern of CXCR4 receptor by immunohistochemistry. Our results show that SDF-1 protein and mRNA are normally expressed by endothelial cells, pericytes, and either resident or explanted CD1a+ dendritic cells. Epithelial cells of sweat glands but not keratinocytes also express SDF-1. In various inflammatory skin diseases, a large number of mononuclear cells and fibroblasts in close contact with CXCR4-positive lymphocytic infiltrates also express SDF-1. CXCR4 was also detected in many different normal cell types, including endothelial and epithelial cells, which points to a role for SDF-1/CXCR4 cell signaling in vascular and epithelial homeostasis. The demonstration of SDF-1 expression in dendritic and endothelial cells provides new insights into the mechanisms of normal and pathological lymphocyte circulation and makes it possible to envisage a role for locally secreted SDF-1 in the selective incapacity of mucosal dendritic cells to support and propagate infection by X4 HIV isolates.
Figures



References
-
- Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 - PubMed
-
- Nagasawa T, Tachibana K, Kawabata K: A CXC chemokine SDF-1/PBSF. A ligand for a HIV receptor, CXCR4. Adv Immunol 1999, 71:211-228 - PubMed
-
- Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T: Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF-1) gene. Genomics 1995, 28:495-500 - PubMed
-
- Nagasawa T, Hirota S, Tachibana K, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T: Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382:635-638 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

