Expression of platelet-derived endothelial cell growth factor and its potential role in up-regulation of angiogenesis in scarred kidneys secondary to urinary tract diseases
- PMID: 10550316
- PMCID: PMC1866986
- DOI: 10.1016/S0002-9440(10)65475-2
Expression of platelet-derived endothelial cell growth factor and its potential role in up-regulation of angiogenesis in scarred kidneys secondary to urinary tract diseases
Abstract
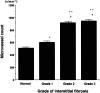
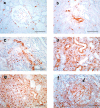
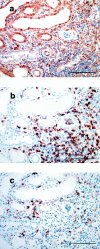
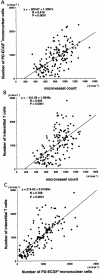
The mechanism of neovascularization secondary to renal interstitial fibrosis is not well understood. Platelet-derived endothelial cell growth factor (PD-ECGF) is known to promote angiogenesis. We examined the expression of PD-ECGF immunohistochemically in 9 normal kidneys and 26 scarred kidneys secondary to urinary tract diseases. To estimate up-regulation of angiogenesis, microvessels were counted by immunostaining endothelial cells for CD34. Immunostaining of PD-ECGF was observed in most Bowman's capsules, occasional tubules, and some interstitial mononuclear cells in normal kidneys. A remarkable increase of immunostained PD-ECGF was found in the tubules and interstitial mononuclear infiltrates in the scarred kidneys. The predominant cell type in the infiltrate was T cells (CD3(+)). The microvessel count and mean numbers of PD-ECGF(+) tubular and interstitial mononuclear cells increased with increasing interstitial fibrosis. A significant correlation was noted between microvessel count and the number of PD-ECGF(+) tubular cells (P = 0.0002) or PD-ECGF(+) interstitial mononuclear cells (P < 0.0001). Immunostaining of endogrin, a marker of endothelial proliferation, increased in the microvessels located in the fibrotic interstitial spaces. These results suggest that angiogenesis may play a critical role in the progression of tubulointerstitial injuries and that up-regulation of PD-ECGF may contribute to neovascularization.
Figures

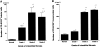



Similar articles
-
Significance of platelet-derived endothelial cell growth factor in the angiogenesis of human gastric cancer.Clin Cancer Res. 1998 Feb;4(2):429-34. Clin Cancer Res. 1998. PMID: 9516932
-
Expression of platelet-derived endothelial cell growth factor correlates with good prognosis in patients with colorectal carcinoma.Cancer. 2000 Jan 1;88(1):42-9. Cancer. 2000. PMID: 10618604
-
Expression of thymidine phosphorylase correlates with microvessel density in prostate cancer.Oncol Rep. 2005 Apr;13(4):597-600. Oncol Rep. 2005. PMID: 15756429
-
Platelet-derived endothelial cell growth factor.J Cell Biochem. 1991 Nov;47(3):208-10. doi: 10.1002/jcb.240470304. J Cell Biochem. 1991. PMID: 1724243 Review.
-
Platelet-derived endothelial cell growth factor: structure and function.Jpn Circ J. 1991 Oct;55(10):1022-6. doi: 10.1253/jcj.55.1022. Jpn Circ J. 1991. PMID: 1744978 Review.
Cited by
-
Proliferation and remodeling of the peritubular microcirculation after nephron reduction: association with the progression of renal lesions.Am J Pathol. 2001 Aug;159(2):547-60. doi: 10.1016/S0002-9440(10)61726-9. Am J Pathol. 2001. PMID: 11485913 Free PMC article.
-
A Putative Role of Apolipoprotein L1 Polymorphism in Renal Parenchymal Scarring Following Febrile Urinary Tract Infection in Nigerian Under-Five Children: Proposal for a Case-Control Association Study.JMIR Res Protoc. 2018 Jun 14;7(6):e156. doi: 10.2196/resprot.9514. JMIR Res Protoc. 2018. PMID: 29903699 Free PMC article.
-
Capillary rarefaction: a missing link in renal and cardiovascular disease?Angiogenesis. 2024 Feb;27(1):23-35. doi: 10.1007/s10456-023-09883-8. Epub 2023 Jun 16. Angiogenesis. 2024. PMID: 37326760 Review.
-
Peritubular capillary rarefaction: a new therapeutic target in chronic kidney disease.Pediatr Nephrol. 2014 Mar;29(3):333-42. doi: 10.1007/s00467-013-2430-y. Epub 2013 Mar 10. Pediatr Nephrol. 2014. PMID: 23475077 Free PMC article. Review.
-
Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha.Am J Pathol. 2003 Dec;163(6):2289-301. doi: 10.1016/s0002-9440(10)63586-9. Am J Pathol. 2003. PMID: 14633603 Free PMC article.
References
-
- Mackensen-Haen S, Bader R, Grund KE, Bohle A: Correlation between renal cortical interstitial fibrosis, atrophy of proximal tubules and impairment of the glomerular filtration rate. Clin Nephrol 1981, 4:167-171 - PubMed
-
- Bohle A, Muler GA, Wehrmann M, Mackensen-Haen S, Xiao JC: Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int 1996, 49:s2-s9 - PubMed
-
- Eddy AA: Experimental insights into the tubulointerstitial disease accompanying primary glomerular lesions. J Am Soc Nephrol 1994, 5:1273-1287 - PubMed
-
- Bohle A, Gise HV, Machensen-Haen S, Stark-Jakob B: The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubuli. Klin Wochenschr 1981, 59:1043-1051 - PubMed
-
- Ong ACM, Fine LG: Loss of glomerular function and tubulointerstitial fibrosis: cause or effect. Kidney Int 1994, 45:345-351 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

