Mouse vascular adhesion protein 1 is a sialoglycoprotein with enzymatic activity and is induced in diabetic insulitis
- PMID: 10550318
- PMCID: PMC1866981
- DOI: 10.1016/S0002-9440(10)65477-6
Mouse vascular adhesion protein 1 is a sialoglycoprotein with enzymatic activity and is induced in diabetic insulitis
Abstract
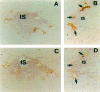
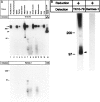
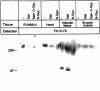
The continuous recirculation of lymphocytes requires an adequate expression and function of the molecules mediating the cellular interactions between endothelium and lymphocytes. Human vascular adhesion protein 1 (hVAP-1) is an endothelial cell adhesion molecule that mediates the binding of lymphocytes to venules in peripheral lymph nodes as well as at sites of inflammation. Recently the mouse homologue of hVAP-1 has been cloned. It is a previously unknown molecule with a significant sequence identity to copper-containing amine oxidases. Besides the sequence, very little is known about the expression, structure, and function of mouse VAP-1 (mVAP-1). In this study we demonstrate that mVAP-1 is prominently expressed in endothelial and smooth muscle (but not in other types of muscle cells), as well as in adipocytes. mVAP-1 is a 220-kd homodimeric sialoglycoprotein that displays cell-type-specific differences in glycosylation. The expression of mVAP-1 is induced on inflammation in the vessels of the endocrine pancreas during the development of insulitis, and the up-regulation correlates with the extent of the lymphocytic infiltrate. In general, different mouse strains displayed very similar VAP-1 expression, but the small differences seen in liver and gut suggest that immunostimulation may modulate VAP-1 synthesis in extrapancreatic organs as well. Finally, we show that mVAP-1 has a monoamine oxidase activity against naturally occurring substrates, implying a role in the development of vasculopathies. These data show that mVAP-1 and hVAP-1 are very similar molecules that nevertheless have certain marked differences in expression, biochemical structure, and substrate specificity. Thus mVAP-1 is a novel inflammation-inducible mouse molecule that has a dual adhesive and enzymatic function.
Figures





Similar articles
-
Cloning and characterization of mouse vascular adhesion protein-1 reveals a novel molecule with enzymatic activity.J Immunol. 1998 Jun 1;160(11):5563-71. J Immunol. 1998. PMID: 9605161
-
Isolation, structural characterization, and chromosomal mapping of the mouse vascular adhesion protein-1 gene and promoter.J Immunol. 1998 Sep 15;161(6):2953-60. J Immunol. 1998. PMID: 9743358
-
Lymphocyte binding to vascular endothelium in inflamed skin revisited: a central role for vascular adhesion protein-1 (VAP-1).Eur J Immunol. 1996 Apr;26(4):825-33. doi: 10.1002/eji.1830260415. Eur J Immunol. 1996. PMID: 8625974
-
A novel endothelial cell molecule mediating lymphocyte binding in humans.Behring Inst Mitt. 1993 Aug;(92):36-43. Behring Inst Mitt. 1993. PMID: 8250815 Review.
-
[Vascular adhesion protein-1 (VAP-1) in inflammatory process].Przegl Lek. 2006;63(4):220-2. Przegl Lek. 2006. PMID: 17083159 Review. Polish.
Cited by
-
Dysregulation of Leukocyte Trafficking in Type 2 Diabetes: Mechanisms and Potential Therapeutic Avenues.Front Cell Dev Biol. 2021 Feb 22;9:624184. doi: 10.3389/fcell.2021.624184. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33692997 Free PMC article. Review.
-
Involvement of semicarbazide-sensitive amine oxidase-mediated deamination in lipopolysaccharide-induced pulmonary inflammation.Am J Pathol. 2006 Mar;168(3):718-26. doi: 10.2353/ajpath.2006.050970. Am J Pathol. 2006. PMID: 16507887 Free PMC article.
-
Expression of vascular adhesion protein-1 in normal and inflamed mice lungs and normal human lungs.Virchows Arch. 2003 May;442(5):491-5. doi: 10.1007/s00428-003-0802-6. Epub 2003 Apr 17. Virchows Arch. 2003. PMID: 12700900
-
Smooth muscle-generated methylglyoxal impairs endothelial cell-mediated vasodilatation of cerebral microvessels in type 1 diabetic rats.Br J Pharmacol. 2016 Dec;173(23):3307-3326. doi: 10.1111/bph.13617. Epub 2016 Oct 14. Br J Pharmacol. 2016. PMID: 27611446 Free PMC article.
-
Vascular adhesion protein-1 and microvascular diabetic complications.Pharmacol Rep. 2022 Feb;74(1):40-46. doi: 10.1007/s43440-021-00343-y. Epub 2022 Jan 10. Pharmacol Rep. 2022. PMID: 35001320 Review.
References
-
- Salmi M, Jalkanen S: How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv Immunol 1997, 64:139-218 - PubMed
-
- Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994, 76:301-314 - PubMed
-
- Salmi M, Jalkanen S: A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 1992, 257:1407-1409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

