Mechanical forces induce scar remodeling. Study in non-pressure-treated versus pressure-treated hypertrophic scars
- PMID: 10550323
- PMCID: PMC1866977
- DOI: 10.1016/S0002-9440(10)65482-X
Mechanical forces induce scar remodeling. Study in non-pressure-treated versus pressure-treated hypertrophic scars
Abstract
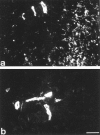
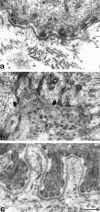
Reparative process of second and third degree burns usually results in hypertrophic scar formation that can be treated by pressure. Although this method is efficient, its mechanisms of action are not known. In this work, we have studied the histological organization of hypertrophic scars submitted to pressure. Skin biopsies were performed 2 to 7 months after the onset of treatment in two adjacent regions of the scar, non-pressure- or pressure-treated and analyzed by immunohistochemistry and transmission electron microscopy for extracellular matrix organization and cellular morphology. In non-pressure-treated regions, fibrillin deposits did not present the classical candelabra-like pattern under epidermis and were reduced in dermis; in pressure-treated regions the amount was increased compared to non-pressure-treated regions but the organization was still disturbed. In non-pressure-treated regions, elastin was present in patch deposits; in pressure-treated regions elastin formed fibers, smaller than in normal dermis. Tenascin was present in the whole dermis in non-pressure-treated regions, whereas in pressure-treated regions it was observed only under epidermis and around vessels, as in normal skin. alpha-Smooth muscle actin-expressing myofibroblasts were absent in normal skin, present in large amounts in non-pressure-treated regions, and almost absent in pressure-treated regions. The disturbed ultrastructural organization of dermal-epidermal junction observed in non-pressure-treated regions disappeared after pressure therapy; typical features of apoptosis in fibroblastic cells and morphological aspects of collagen degradation were observed in pressure-treated regions. Our results show that, in hypertrophic scars, pressure therapy restores in part the extracellular matrix organization observed in normal scar and induces the disappearance of alpha-smooth muscle actin-expressing myofibroblasts, probably by apoptosis. We suggest that the pressure acts by accelerating the remission phase of the postburn reparative process.
Figures




Similar articles
-
A histological study on the effect of pressure therapy on the activities of myofibroblasts and keratinocytes in hypertrophic scar tissues after burn.Burns. 2015 Aug;41(5):1008-16. doi: 10.1016/j.burns.2014.11.017. Epub 2015 Feb 11. Burns. 2015. PMID: 25681960
-
Epidermis promotes dermal fibrosis: role in the pathogenesis of hypertrophic scars.J Pathol. 2005 May;206(1):1-8. doi: 10.1002/path.1737. J Pathol. 2005. PMID: 15772942
-
Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids.Wound Repair Regen. 2004 Mar-Apr;12(2):169-74. doi: 10.1111/j.1067-1927.2004.012209.x. Wound Repair Regen. 2004. PMID: 15086768
-
Control of wound contraction. Basic and clinical features.Hand Clin. 2000 May;16(2):289-302. Hand Clin. 2000. PMID: 10791174 Review.
-
Potential cellular and molecular causes of hypertrophic scar formation.Burns. 2009 Feb;35(1):15-29. doi: 10.1016/j.burns.2008.06.020. Epub 2008 Oct 25. Burns. 2009. PMID: 18952381 Review.
Cited by
-
The micrograft concept for wound healing: strategies and applications.J Diabetes Sci Technol. 2010 Jul 1;4(4):808-19. doi: 10.1177/193229681000400407. J Diabetes Sci Technol. 2010. PMID: 20663442 Free PMC article. Review.
-
Use of tape for the management of hypertrophic scar development: A comprehensive review.Scars Burn Heal. 2021 Jul 12;7:20595131211029206. doi: 10.1177/20595131211029206. eCollection 2021 Jan-Dec. Scars Burn Heal. 2021. PMID: 34290886 Free PMC article. Review.
-
A novel self-inflatable balloon for treating refractory benign esophageal strictures: a prospective, single-arm, multicenter study.Int J Surg. 2024 Apr 1;110(4):2055-2064. doi: 10.1097/JS9.0000000000001120. Int J Surg. 2024. PMID: 38668658 Free PMC article.
-
Scar formation following excisional and burn injuries in a red Duroc pig model.Wound Repair Regen. 2017 Aug;25(4):618-631. doi: 10.1111/wrr.12562. Epub 2017 Jul 31. Wound Repair Regen. 2017. PMID: 28727221 Free PMC article.
-
Update on hypertrophic scar treatment.Clinics (Sao Paulo). 2014 Aug;69(8):565-73. doi: 10.6061/clinics/2014(08)11. Clinics (Sao Paulo). 2014. PMID: 25141117 Free PMC article. Review.
References
-
- Linares HA: Pathophysiology of the burn scar. Herndon DN eds. Total Burn Care. 1996, :pp 383-397 Saunders, London
-
- Gabbiani G, Ryan GB, Majno G: Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27:549-550 - PubMed
-
- Desmoulière A, Gabbiani G: The role of the myofibroblast in wound healing and fibrocontractive diseases. Clark RAF eds. The Molecular and Cellular Biology of Wound Repair. 1996, :pp 391-423 Plenum Press, New York
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

