Motility induced by human immunodeficiency virus-1 Tat on Kaposi's sarcoma cells requires platelet-activating factor synthesis
- PMID: 10550329
- PMCID: PMC1866979
- DOI: 10.1016/S0002-9440(10)65488-0
Motility induced by human immunodeficiency virus-1 Tat on Kaposi's sarcoma cells requires platelet-activating factor synthesis
Abstract
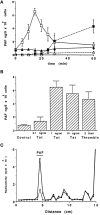
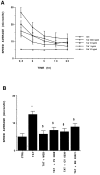
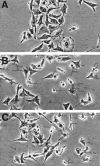
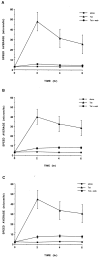
In the present study, we evaluated whether motility of Kaposi's sarcoma (KS) spindle cells induced by HIV-1 Tat protein is dependent on the synthesis of platelet-activating factor (PAF). The results obtained indicate that Tat induced a dose-dependent synthesis of PAF from KS cells at a concentration as low as 0.1 ng/ml. PAF production started rapidly after Tat stimulation, peaking at 30 minutes and declining thereafter. Tat-induced cell migration was also a rapid event starting at 30 minutes. The motility was abrogated by addition of a panel of chemically unrelated PAF receptor antagonists (WEB 2170, CV 3988, CV 6209, and BN 52021), suggesting that the synthesized PAF mediates the motogenic effect of Tat. This effect was also present on cells plated on a type-I collagen-, fibronectin-, or basement membrane extract-coated surface. Expression of PAF receptor-specific mRNA was detected in KS cells. In addition, examination of the cytoskeletal organization showed that Tat-mediated KS cell redistribution of actin filaments and shape change was also inhibited by a PAF receptor antagonist. Moreover, PAF receptor blockade prevented the up-regulation of beta1 integrin and the down-regulation of alphavbeta3 observed after stimulation of KS cells with Tat. In conclusion, the results of the present study indicate that Tat-induced PAF synthesis plays a critical role in triggering the events involved in motility of KS cells.
Figures



Similar articles
-
The synthesis of platelet-activating factor modulates chemotaxis of monocytes induced by HIV-1 Tat.Eur J Immunol. 1999 May;29(5):1513-21. doi: 10.1002/(SICI)1521-4141(199905)29:05<1513::AID-IMMU1513>3.0.CO;2-X. Eur J Immunol. 1999. PMID: 10359105
-
Platelet activating factor produced in vitro by Kaposi's sarcoma cells induces and sustains in vivo angiogenesis.J Clin Invest. 1995 Aug;96(2):940-52. doi: 10.1172/JCI118142. J Clin Invest. 1995. PMID: 7543496 Free PMC article.
-
Sensitization of human neutrophil defense activities through activation of platelet-activating factor receptors by ginkgolide B, a bioactive component of the Ginkgo biloba extract EGB 761.Biochem Pharmacol. 2002 Apr 1;63(7):1241-9. doi: 10.1016/s0006-2952(01)00866-8. Biochem Pharmacol. 2002. PMID: 11960600
-
Therapy for acute pancreatitis with platelet-activating factor receptor antagonists.World J Gastroenterol. 2008 Aug 14;14(30):4735-8. doi: 10.3748/wjg.14.4735. World J Gastroenterol. 2008. PMID: 18720532 Free PMC article. Review.
-
HIV-Tat dependent chemotaxis and invasion, key aspects of tat mediated pathogenesis.Clin Exp Metastasis. 2000;18(7):533-8. doi: 10.1023/a:1011991906685. Clin Exp Metastasis. 2000. PMID: 11688957 Review.
Cited by
-
Platelet-activating factor regulates cadherin-catenin adhesion system expression and beta-catenin phosphorylation during Kaposi's sarcoma cell motility.Am J Pathol. 2005 May;166(5):1515-22. doi: 10.1016/s0002-9440(10)62367-x. Am J Pathol. 2005. PMID: 15855650 Free PMC article.
-
PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis.Am J Pathol. 2000 Nov;157(5):1713-25. doi: 10.1016/S0002-9440(10)64808-0. Am J Pathol. 2000. PMID: 11073830 Free PMC article.
-
Human immunodeficiency virus-1 tat induces hyperproliferation and dysregulation of renal glomerular epithelial cells.Am J Pathol. 2002 Jul;161(1):53-61. doi: 10.1016/S0002-9440(10)64156-9. Am J Pathol. 2002. PMID: 12107089 Free PMC article.
-
Palliation of bone cancer pain by antagonists of platelet-activating factor receptors.PLoS One. 2014 Mar 17;9(3):e91746. doi: 10.1371/journal.pone.0091746. eCollection 2014. PLoS One. 2014. PMID: 24637403 Free PMC article.
-
Proliferative activity of extracellular HIV-1 Tat protein in human epithelial cells: expression profile of pathogenetically relevant genes.BMC Microbiol. 2005 Apr 27;5:20. doi: 10.1186/1471-2180-5-20. BMC Microbiol. 2005. PMID: 15857508 Free PMC article.
References
-
- Ensoli B, Barillari G, Gallo RC: Cytokine and growth factors in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Immunol Rev 1992, 127:147-155 - PubMed
-
- Masood R, Cai J, Law R, Gill P: AIDS-associated Kaposi’s sarcoma pathogenesis, clinical features, and treatment. J Opin Oncol 1993, 5:831-834 - PubMed
-
- Rabkin CS, Janz S, Lash A, Coleman AE, Musaba E, Liotta L, Biggar R, Zhuang Z: Monoclonal origin of multicentric Kaposi’s sarcoma lesions. N Engl J Med 1997, 336:988-993 - PubMed
-
- Ensoli B, Barillari G, Buonaguro L, Gallo RC: Molecular mechanisms in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Adv Exp Med Biol 1991, 303:27-38 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

