Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells
- PMID: 10550331
- PMCID: PMC1866993
- DOI: 10.1016/S0002-9440(10)65490-9
Lipopolysaccharide-activated macrophages stimulate the synthesis of collagen type I and C-fibronectin in cultured pancreatic stellate cells
Abstract
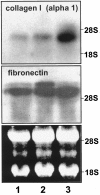
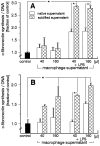
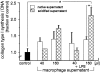
We have recently identified and characterized pancreatic stellate cells (PSC) in rats and humans (Gastroenterology 1998, 15:421-435). PSC are suggested to represent the main cellular source of extracellular matrix in chronic pancreatitis. Now we describe a paracrine stimulatory loop between human macrophages and PSC (rat and human) that results in an increased extracellular matrix synthesis. Native and transiently acidified supernatants of cultured macrophages were added to cultured PSC in the presence of 0.1% fetal calf serum. Native supernatants of lipopolysaccharide-activated macrophages stimulated the synthesis of collagen type I 1.38 +/- 0.09-fold of control and c-fibronectin 1.89 +/- 0.18-fold of control. Transiently acidified supernatants stimulated collagen type I and c-fibronectin 2.10 +/- 0.2-fold and 2.80 +/- 0.05-fold of control, respectively. Northern blot demonstrated an increased expression of the collagen-I-(alpha-1)-mRNA and fibronectin-mRNA in PSC 10 hours after addition of the acidified macrophage supernatants. Cell proliferation measured by bromodeoxyuridine incorporation was not influenced by the macrophage supernatants. Unstimulated macrophages released 1.97 pg TGFbeta1/microgram of DNA over 24 hours and lipopolysaccharide-activated macrophages released 6.61pg TGFbeta1/microgram of DNA over 24 hours. These data together with the results that, in particular, transiently acidified macrophage supernatants increased matrix synthesis, identify TGFbeta as the responsible mediator. In conclusion, our data demonstrate a paracrine stimulation of matrix synthesis of pancreatic stellate cells via TGFbeta1 released by activated macrophages. We suggest that macrophages might play a pivotal role in the development of pancreas fibrosis.
Figures

References
-
- Sarles H, Bernard JP, Johnson C: Pathogenesis and epidemiology of chronic pancreatitis. Ann Rev Med 1989, 40:453-468 - PubMed
-
- Morohoshi T, Kanda M: Periacinar fibroblastoid cell: its action on early stage of alcoholic pancreatitis. J Biliary Tract Pancreas 1985, 6:1205-1211(in Japanese)
-
- Di Magno EP, Layer P, Clain JE: Chronic pancreatitis. 21st ed. Go VWL Di Magno EP Gardner JD eds. The Pancreas, 1993, :pp 655-706 Raven Press, New York
-
- Elsässer HP, Adler G, Kern HF: Fibroblast structure and function during regeneration from hormone-induced acute pancreatitis in the rat. Pancreas 1989, 4:169-178 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

