Postsynaptic expression of tetanus toxin light chain blocks synaptogenesis in Drosophila
- PMID: 10556094
- PMCID: PMC2726806
- DOI: 10.1016/s0960-9822(99)80510-7
Postsynaptic expression of tetanus toxin light chain blocks synaptogenesis in Drosophila
Abstract
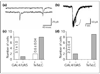
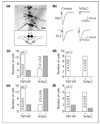
During the development of the nervous system embryonic neurons are incorporated into neural networks that underlie behaviour. For example, during embryogenesis in Drosophila, motor neurons in every body segment are wired into the circuitry that drives the simple peristaltic locomotion of the larva. Very little is known about the way in which the necessary central synapses are formed in such a network or how their properties are controlled. One possibility is that presynaptic and postsynaptic elements form relatively independently of each other. Alternatively, there might be an interaction between presynaptic and postsynaptic neurons that allows for adjustment and plasticity in the embryonic network. Here we have addressed this issue by analysing the role of synaptic transmission in the formation of synaptic inputs onto identified motorneurons as the locomotor circuitry is assembled in the Drosophila embryo. We targeted the expression of tetanus toxin light chain (TeTxLC) to single identified neurons using the GAL4 system. TeTxLC prevents the evoked release of neurotransmitter by enzymatically cleaving the synaptic-vesicle-associated protein neuronal-Synaptobrevin (n-Syb) [1]. Unexpectedly, we found that the cells that expressed TeTxLC, which were themselves incapable of evoked release, showed a dramatic reduction in synaptic input. We detected this reduction both electrophysiologically and ultrastructurally.
Figures


References
-
- Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. - PubMed
-
- Mlodzik M, Baker NE, Rubin GM. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990;4:1848–1861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

