Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation
- PMID: 10556918
- PMCID: PMC1571708
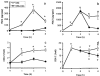
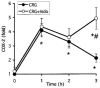
- DOI: 10.1038/sj.bjp.0702866
Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation
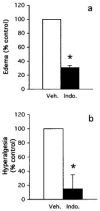
Abstract
1 We characterized the regulation of cyclooxygenase-2 (COX-2) at the mRNA, protein and mediator level in two rat models of acute inflammation, carrageenan-induced paw oedema and mechanical hyperalgesia. 2 Carrageenan was injected in the hind paw of rat at low (paw oedema) and high doses (hyperalgesia). COX-2 and prostaglandin E2 (PGE2) levels were measured by RT-PCR and immunological assays. We also determined the distribution of COX-2 by immunohistochemistry. 3 The injection of carrageenan produced a significant and parallel induction of both COX-2 and PGE2. This induction was significantly higher in hyperalgesia than in paw oedema. This was probably due to the 9 fold higher concentration of carrageenan used to provoke hyperalgesia. 4 Immunohistochemical examination showed COX-2 immunoreactivity in the epidermis, skeletal muscle and inflammatory cells of rats experiencing hyperalgesia. In paw oedema however, only the epidermis showed positive COX-2 immunoreactivity. 5 Pretreatment with indomethacin completely abolished the induction of COX-2 in paw oedema but not in hyperalgesia. 6 These results suggest that multiple mechanisms regulate COX-2 induction especially in the more severe model. In carrageenan-induced paw oedema, prostanoid production have been linked through the expression of the COX-2 gene which suggest the presence of a positive feedback loop mechanism.
Figures
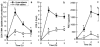

Similar articles
-
Cyclooxygenase-2 is induced in the endothelial cells throughout the central nervous system during carrageenan-induced hind paw inflammation; its possible role in hyperalgesia.J Neurochem. 2003 Jul;86(2):318-28. doi: 10.1046/j.1471-4159.2003.01848.x. J Neurochem. 2003. PMID: 12871573
-
Selective inhibitors of cyclo-oxygenase-2 (COX-2) induce hypoalgesia in a rat paw model of inflammation.Br J Pharmacol. 2002 Nov;137(6):837-44. doi: 10.1038/sj.bjp.0704937. Br J Pharmacol. 2002. PMID: 12411415 Free PMC article.
-
Pharmacological analysis of cyclooxygenase-1 in inflammation.Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13313-8. doi: 10.1073/pnas.95.22.13313. Proc Natl Acad Sci U S A. 1998. PMID: 9789085 Free PMC article.
-
Effect of the selective COX-2 inhibitors, celecoxib and rofecoxib in rat acute models of inflammation.Inflamm Res. 2002 Dec;51(12):603-10. doi: 10.1007/pl00012435. Inflamm Res. 2002. PMID: 12558194
-
Biochemical and pharmacological profile of a tetrasubstituted furanone as a highly selective COX-2 inhibitor.Br J Pharmacol. 1997 May;121(1):105-17. doi: 10.1038/sj.bjp.0701076. Br J Pharmacol. 1997. PMID: 9146894 Free PMC article.
Cited by
-
Alloferon-1 ameliorates acute inflammatory responses in λ-carrageenan-induced paw edema in mice.Sci Rep. 2022 Oct 6;12(1):16689. doi: 10.1038/s41598-022-20648-z. Sci Rep. 2022. PMID: 36202869 Free PMC article.
-
The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations.Metab Brain Dis. 2022 Apr;37(4):859-880. doi: 10.1007/s11011-022-00960-3. Epub 2022 Mar 25. Metab Brain Dis. 2022. PMID: 35334041 Review.
-
Acute changes in muscle blood flow and concomitant muscle damage after an intramuscular administration.Pharm Res. 2005 Mar;22(3):405-12. doi: 10.1007/s11095-004-1878-7. Pharm Res. 2005. PMID: 15835746
-
Analgesic and Anti-Inflammatory Activities of the Methanol Extract from Pogostemon cablin.Evid Based Complement Alternat Med. 2011;2011:671741. doi: 10.1093/ecam/nep183. Epub 2011 Feb 14. Evid Based Complement Alternat Med. 2011. PMID: 19933324 Free PMC article.
-
Anti-inflammatory and anti-nociceptive activities of methanol extract from aerial part of Phlomis younghusbandii Mukerjee.PLoS One. 2014 Mar 5;9(3):e89149. doi: 10.1371/journal.pone.0089149. eCollection 2014. PLoS One. 2014. PMID: 24598860 Free PMC article.
References
-
- ALMEIDA A.P., BAYER B.M., HORAKOVA Z., BEAVEN M.A. Influence of indomethacin and other anti-inflammatory drugs on mobilization and production of neutrophils: studies with carrageenan-id inflammation in rats. J. Pharmacol. Exp. Ther. 1980;214:74–79. - PubMed
-
- ARIAS-NEGRETE S., KELLER K., CHADEE K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem. Biophys. Res. Commun. 1995;208:582–589. - PubMed
-
- BJARNASON I., MACPHERSON A., ROTMAN H., SCHUPP J., HAYLLAR J. A randomized, double-blind, crossover comparative endoscopy study on the gastroduodenal tolerability of a highly specific cyclooxygenase-2 inhibitor, flosulide, and naproxen. Scand. J. Gastroenterol. 1997;32:126–130. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

