Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene
- PMID: 10557207
- PMCID: PMC317121
- DOI: 10.1101/gad.13.21.2787
Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene
Abstract
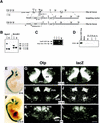
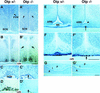
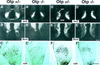
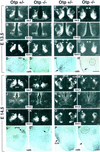
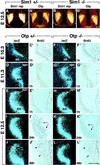
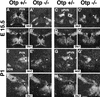
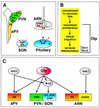
Development of the neuroendocrine hypothalamus is characterized by a precise series of morphogenetic milestones culminating in terminal differentiation of neurosecretory cell lineages. The homeobox-containing gene Orthopedia (Otp) is expressed in neurons giving rise to the paraventricular (PVN), supraoptic (SON), anterior periventricular (aPV), and arcuate (ARN) nuclei throughout their development. Homozygous Otp(-/-) mice die soon after birth and display progressive impairment of crucial neuroendocrine developmental events such as reduced cell proliferation, abnormal cell migration, and failure in terminal differentiation of the parvocellular and magnocellular neurons of the aPV, PVN, SON, and ARN. Moreover, our data provide evidence that Otp and Sim1, a bHLH-PAS transcription factor that directs terminal differentiation of the PVN, SON, and aPV, act in parallel and are both required to maintain Brn2 expression which, in turn, is required for neuronal cell lineages secreting oxytocin (OT), arginine vasopressin (AVP), and corticotropin-releasing hormone (CRH).
Figures


References
-
- Acampora D, Mazan S, Tuorto F, Avantaggiato V, Tremblay JJ, Lazzaro D, di Carlo A, Mariano A, Macchia PE, Corte G, et al. Transient dwarfism and hypogonadism in mice lacking Otx1 reveal prepubescent stage-specific control of pituitary levels of GH, FSH and LH. Development. 1998;125:1229–1239. - PubMed
-
- Alvarez-Bolado G, Rosenfeld MG, Swanson LW. Model of forebrain regionalization based on spatiotemporal patterns of POU-III homeobox gene expression, birthdates, and morphological features. J Comp Neurol. 1995;355:237–295. - PubMed
-
- Bach I, Carrière C, Ostendorff HP, Andersen B, Rosenfeld MG. A family of LIM domain-associated cofactors confer transcriptional synergism between LIM and Otx homeodomain proteins. Genes & Dev. 1997;11:1370–1380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
