Mice lacking both presenilin genes exhibit early embryonic patterning defects
- PMID: 10557208
- PMCID: PMC317124
- DOI: 10.1101/gad.13.21.2801
Mice lacking both presenilin genes exhibit early embryonic patterning defects
Abstract
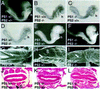
Genetic studies in worms, flies, and humans have implicated the presenilins in the regulation of the Notch signaling pathway and in the pathogenesis of Alzheimer's Disease. There are two highly homologous presenilin genes in mammals, presenilin 1 (PS1) and presenilin 2 (PS2). In mice, inactivation of PS1 leads to developmental defects that culminate in a perinatal lethality. To test the possibility that the late lethality of PS1-null mice reflects genetic redundancy of the presenilins, we have generated PS2-null mice by gene targeting, and subsequently, PS1/PS2 double-null mice. Mice homozygous for a targeted null mutation in PS2 exhibit no obvious defects; however, loss of PS2 on a PS1-null background leads to embryonic lethality at embryonic day 9.5. Embryos lacking both presenilins, and surprisingly, those carrying only a single copy of PS2 on a PS1-null background, exhibit multiple early patterning defects, including lack of somite segmentation, disorganization of the trunk ventral neural tube, midbrain mesenchyme cell loss, anterior neuropore closure delays, and abnormal heart and second branchial arch development. In addition, Delta like-1 (Dll1) and Hes-5, two genes that lie downstream in the Notch pathway, were misexpressed in presenilin double-null embryos: Hes-5 expression was undetectable in these mice, whereas Dll1 was expressed ectopically in the neural tube and brain of double-null embryos. We conclude that the presenilins play a widespread role in embryogenesis, that there is a functional redundancy between PS1 and PS2, and that both vertebrate presenilins, like their invertebrate homologs, are essential for Notch signaling.
Figures





References
-
- Akanawa C, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem. 1992;267:21879–21885. - PubMed
-
- Anafi M, Kiefer F, Gish GD, Mbamalu G, Iscove NN, Pawson T. SH2/SH3 adaptor proteins can link tyrosine kinases to a ste20-related protein kinase, HPK1. J Biol Chem. 1997;272:27804–27811. - PubMed
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: Cell fate control and signal integration in development. Science. 1999;284:770–776. - PubMed
-
- Baumeister R, Leimer U, Zweckbronner I, Jakubek C, Grunberg J, Haass C. Human presenilin-1, but not familial Alzheimer's disease (FAD) mutants, facilitate Caenorhabditis elegans Notch signalling independently of proteolytic processing. Genes Funct. 1997;1:149–159. - PubMed
-
- Berezovska O, Xia MQ, Hyman BT. Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:738–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
