Expression of endoxyloglucan transferase genes in acaulis mutants of Arabidopsis
- PMID: 10557219
- PMCID: PMC59433
- DOI: 10.1104/pp.121.3.715
Expression of endoxyloglucan transferase genes in acaulis mutants of Arabidopsis
Abstract
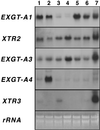
A mutant of Arabidopsis with reduced internodal cell length, acaulis5 (acl5), has recently been shown to have reduced transcript levels of a gene for endoxyloglucan transferase, EXGT-A1 (Y. Hanzawa, T. Takahashi, Y. Komeda [1997] Plant J 12: 863-874). In the present study, we cloned genomic fragments of five members of the EXGT gene family, EXGT-A1, EXGT-A3, EXGT-A4, XTR2, and XTR3, and examined their expression in the wild type and in a series of acl mutants. In wild-type plants, the EXGT-A3 gene showed higher expression in lower internodes (internodes between nodes bearing axillary shoots) than in upper and young internodes, in which EXGT-A1 was highly expressed. EXGT-A4 was preferentially expressed in roots and XTR3 in siliques. The XTR2 gene was constitutively expressed. In acl1, acl3, and acl4 mutants, which have a severe defect in leaf expansion as well as in internode elongation, the EXGT-A1 gene showed reduced levels of expression before bolting of plants. In contrast, XTR3 was increased in these mutant seedlings. Reduction of EXGT-A1 expression was also detected after bolting of all acl mutants except acl2, whose growth defect is restricted to lower internodes. These results suggest the involvement of each EXGT in different aspects of organ development.
Figures





References
-
- Arrowsmith DA, de Silva J. Characterisation of two tomato fruit-expressed cDNAs encoding xyloglucan endo-transglycosylase. Plant Mol Biol. 1995;28:391–403. - PubMed
-
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. - PubMed
-
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993;3:1–30. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

