Okadaic Acid Mimics Nitrogen-Stimulated Transcription of the NADH-Glutamate Synthase Gene in Rice Cell Cultures
- PMID: 10557228
- PMCID: PMC59442
- DOI: 10.1104/pp.121.3.805
Okadaic Acid Mimics Nitrogen-Stimulated Transcription of the NADH-Glutamate Synthase Gene in Rice Cell Cultures
Abstract
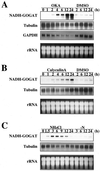
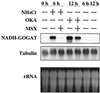
Okadaic acid (OKA), a potent and specific inhibitor of protein serine/threonine phosphatases 1 and 2A, induced the accumulation of NADH-glutamate synthase (GOGAT) mRNA within 4 h in rice (Oryza sativa L.) cell cultures. In contrast to the transient accumulation of NADH-GOGAT mRNA by NH(4)(+), OKA caused a continuous accumulation for at least 24 h. The induction of NADH-GOGAT mRNA by OKA was not inhibited in the presence of methionine sulfoximine, which inhibited the NH(4)(+)-induced accumulation of mRNA. These results suggest that the OKA-sensitive protein phosphatase is involved in the regulation of NADH-GOGAT gene expression and probably plays a role in the signal transduction pathway downstream from NH(4)(+), although a signal transduction pathway other than that of nitrogen sensing could be responsible. Nuclear run-on assays demonstrated that the accumulation of NADH-GOGAT mRNA induced by the supply of either NH(4)(+) or OKA was mainly regulated at the transcription level. OKA effects were synergistic to the NH(4)(+)-induced expression of the NADH-GOGAT gene. In the presence of K-252a, a protein kinase inhibitor, the accumulation of NADH-GOGAT mRNA induced by either NH(4)(+) or OKA was reduced. The possible roles of protein phosphatases in the regulation of NADH-GOGAT gene expression are discussed.
Figures



Similar articles
-
Organization and structure of NADH-dependent glutamate synthase gene from rice plants.Biochim Biophys Acta. 1998 Sep 8;1387(1-2):298-308. doi: 10.1016/s0167-4838(98)00142-3. Biochim Biophys Acta. 1998. PMID: 9748637
-
Expression of NADH-dependent glutamate synthase protein in the epidermis and exodermis of rice roots in response to the supply of ammonium ions.Planta. 1998 Mar;204(3):288-94. doi: 10.1007/s004250050258. Planta. 1998. PMID: 9530872
-
Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice.J Exp Bot. 2002 Apr;53(370):917-25. doi: 10.1093/jexbot/53.370.917. J Exp Bot. 2002. PMID: 11912234 Review.
-
Transcriptional induction of cyclooxygenase-2 gene by okadaic acid inhibition of phosphatase activity in human chondrocytes: co-stimulation of AP-1 and CRE nuclear binding proteins.J Cell Biochem. 1998 Jun 15;69(4):392-413. J Cell Biochem. 1998. PMID: 9620167
-
Cellular compartmentation of ammonium assimilation in rice and barley.J Exp Bot. 2001 Apr;52(356):591-604. J Exp Bot. 2001. PMID: 11373307 Review.
Cited by
-
Coregulation of glutamine synthetase1;2 (GLN1;2) and NADH-dependent glutamate synthase (GLT1) gene expression in Arabidopsis roots in response to ammonium supply.Front Plant Sci. 2023 Feb 20;14:1127006. doi: 10.3389/fpls.2023.1127006. eCollection 2023. Front Plant Sci. 2023. PMID: 36890884 Free PMC article.
-
Transcriptional downregulation of rice rpL32 gene under abiotic stress is associated with removal of transcription factors within the promoter region.PLoS One. 2011;6(11):e28058. doi: 10.1371/journal.pone.0028058. Epub 2011 Nov 23. PLoS One. 2011. PMID: 22132208 Free PMC article.
-
Genome-Wide Comprehensive Analysis of the Nitrogen Metabolism Toolbox Reveals Its Evolution and Abiotic Stress Responsiveness in Rice (Oryza sativa L.).Int J Mol Sci. 2022 Dec 24;24(1):288. doi: 10.3390/ijms24010288. Int J Mol Sci. 2022. PMID: 36613735 Free PMC article.
-
Glutamine Synthetase and Glutamate Synthase Family Perform Diverse Physiological Functions in Exogenous Hormones and Abiotic Stress Responses in Pyrus betulifolia Bunge (P.be).Plants (Basel). 2024 Oct 1;13(19):2759. doi: 10.3390/plants13192759. Plants (Basel). 2024. PMID: 39409629 Free PMC article.
-
Regulatory role of indeterminate domain 10 (IDD10) in ammonium-dependent gene expression in rice roots.Plant Signal Behav. 2013 May;8(5):e24139. doi: 10.4161/psb.24139. Epub 2013 Mar 7. Plant Signal Behav. 2013. PMID: 23470720 Free PMC article.
References
-
- Avila C, Márquetz AJ, Pajuelo P, Cannell ME, Wallsgrove RM, Forde BG. Cloning and sequence analysis of a cDNA for barley ferredoxin-dependent glutamate synthase and molecular analysis of photorespiratory mutants deficient in the enzyme. Planta. 1993;189:475–483. - PubMed
-
- Cohen P, Holmes CFB, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

