Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice
- PMID: 10557230
- PMCID: PMC59444
- DOI: 10.1104/pp.121.3.821
Cloning and functional expression of a cytochrome P450 cDNA encoding 2-hydroxyisoflavanone synthase involved in biosynthesis of the isoflavonoid skeleton in licorice
Abstract
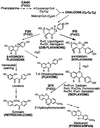
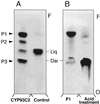
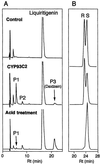
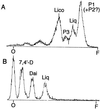
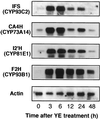
Isoflavonoids are distributed predominantly in leguminous plants and play critical roles in plant physiology. A cytochrome P450 (P450), 2-hydroxyisoflavanone synthase, is the key enzyme in their biosynthesis. In cultured licorice (Glycyrrhiza echinata L., Fabaceae) cells, the production of both an isoflavonoid-derived phytoalexin (medicarpin) and a retrochalcone (echinatin) is rapidly induced upon elicitation. In this study, we obtained a full-length P450 cDNA, CYP Ge-8 (CYP93C2), from the cDNA library of elicited G. echinata cells. When the flavanones liquiritigenin and naringenin were incubated with the recombinant yeast microsome expressing CYP93C2, major products emerged and were readily converted to the isoflavones daidzein and genistein by acid treatment. The chemical structures of the products from liquiritigenin (2-hydroxyisoflavanone and isoflavone) were confirmed by mass spectrometry. CYP93C2 was thus shown to encode 2-hydroxyisoflavanone synthase, which catalyzes the hydroxylation associated with 1,2-aryl migration of flavanones. Northern-blot analysis revealed that transcripts of CYP93C2, in addition to those of other P450s involved in phenylpropanoid/flavonoid pathways, transiently accumulate upon elicitation.
Figures



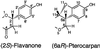
References
-
- Akashi T, Aoki T, Ayabe S. CYP81E1, a cytochrome P450 cDNA of licorice (Glycyrrhiza echinataL.), encodes isoflavone 2′-hydroxylase. Biochem Biophys Res Commun. 1998a;251:67–70. - PubMed
-
- Akashi T, Aoki T, Ayabe S. Identification of a cytochrome P450 cDNA encoding (2S)-flavanone 2-hydroxylase of licorice (Glycyrrhiza echinataL.; Fabaceae) which represents licodione synthase and flavone synthase II. FEBS Lett. 1998b;431:287–290. - PubMed
-
- Akashi T, Aoki T, Takahashi T, Kameya N, Nakamura I, Ayabe S. Cloning of cytochrome P450 cDNAs from cultured Glycyrrhiza echinataL. cells and their transcriptional activation by elicitor-treatment. Plant Sci. 1997b;126:39–47.
-
- Ayabe S, Iida K, Furuya T. Stress-induced formation of echinatin and a metabolite, prenyl-licodione, in cultured Glycyrrhiza echinatacells. Phytochemistry. 1986;25:2803–2806.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

