AXR1 acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis
- PMID: 10557232
- PMCID: PMC59446
- DOI: 10.1104/pp.121.3.839
AXR1 acts after lateral bud formation to inhibit lateral bud growth in Arabidopsis
Abstract
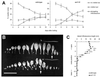
The AXR1 gene of Arabidopsis is required for many auxin responses. The highly branched shoot phenotype of mature axr1 mutant plants has been taken as genetic evidence for a role of auxin in the control of shoot branching. We compared the development of lateral shoots in wild-type Columbia and axr1-12 plants. In the wild type, the pattern of lateral shoot development depends on the developmental stage of the plant. During prolonged vegetative growth, axillary shoots arise and develop in a basal-apical sequence. After floral transition, axillary shoots arise rapidly along the primary shoot axis and grow out to form lateral inflorescences in an apical-basal sequence. For both patterns, the axr1 mutation does not affect the timing of axillary meristem formation; however, subsequent lateral shoot development proceeds more rapidly in axr1 plants. The outgrowth of lateral inflorescences from excised cauline nodes of wild-type plants is inhibited by apical auxin. axr1-12 nodes are resistant to this inhibition. These results provide evidence for common control of axillary growth in both patterns, and suggest a role for auxin during the late stages of axillary shoot development following the formation of the axillary bud and several axillary leaf primordia.
Figures


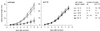
References
-
- Abel S, Nguyen MD, Theologis A. The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol. 1995;251:533–549. - PubMed
-
- Alvarez J, Guli CL, Yu X-H, Smyth DR. terminal flower: a gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 1992;2:103–116.
-
- Cline MG. Apical dominance. Bot Rev. 1991;57:318–358.
-
- Cline MG. The role of hormones in apical dominance: new approaches to an old problem in plant development. Physiol Plant. 1994;90:230–237.
-
- Cline MG. Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot. 1996;78:255–266.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

