Evaluation of functional interaction between K(+) channel alpha- and beta-subunits and putative inactivation gating by Co-expression in Xenopus laevis oocytes
- PMID: 10557249
- PMCID: PMC59464
- DOI: 10.1104/pp.121.3.995
Evaluation of functional interaction between K(+) channel alpha- and beta-subunits and putative inactivation gating by Co-expression in Xenopus laevis oocytes
Abstract
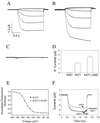
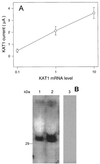
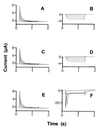
Animal K(+) channel alpha- (pore-forming) subunits form native proteins by association with beta-subunits, which are thought to affect channel function by modifying electrophysiological parameters of currents (often by inducing fast inactivation) or by stabilizing the protein complex. We evaluated the functional association of KAT1, a plant K(+) channel alpha-subunit, and KAB1 (a putative homolog of animal K(+) channel beta-subunits) by co-expression in Xenopus laevis oocytes. Oocytes expressing KAT1 displayed inward-rectifying, non-inactivating K(+) currents that were similar in magnitude to those reported in prior studies. K(+) currents recorded from oocytes expressing both KAT1 and KAB1 had similar gating kinetics. However, co-expression resulted in greater total current, consistent with the possibility that KAB1 is a beta-subunit that stabilizes and therefore enhances surface expression of K(+) channel protein complexes formed by alpha-subunits such as KAT1. K(+) channel protein complexes formed by alpha-subunits such as KAT1 that undergo (voltage-dependent) inactivation do so by means of a "ball and chain" mechanism; the ball portion of the protein complex (which can be formed by the N terminus of either an alpha- or beta-subunit) occludes the channel pore. KAT1 was co-expressed in oocytes with an animal K(+) channel alpha-subunit (hKv1.4) known to contain the N-terminal ball and chain. Inward currents through heteromeric hKv1. 4:KAT1 channels did undergo typical voltage-dependent inactivation. These results suggest that inward currents through K(+) channel proteins formed at least in part by KAT1 polypeptides are capable of inactivation, but the structural component facilitating inactivation is not present when channel complexes are formed by either KAT1 or KAB1 in the absence of additional subunits.
Figures

References
-
- Cao Y, Crawford NM, Schroeder JI. Amino terminus and the first four membrane-spanning segments of the Arabidopsis K+ channel KAT1 confer inward-rectification property of plant-animal chimeric channels. J Biol Chem. 1995;270:17697–17701. - PubMed
-
- Dolly JO, Rettig J, Scott VES, Parcej DN, Wittka R, Sewing S, Pongs O. Oligomeric and subunit structures of neuronal voltage-sensitive K+ channels. Biochem Soc Trans. 1994;22:473–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

