Geranyl diphosphate synthase: cloning, expression, and characterization of this prenyltransferase as a heterodimer
- PMID: 10557273
- PMCID: PMC23900
- DOI: 10.1073/pnas.96.23.13062
Geranyl diphosphate synthase: cloning, expression, and characterization of this prenyltransferase as a heterodimer
Abstract
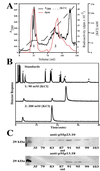
Geranyl diphosphate synthase, which catalyzes the condensation of dimethylallyl diphosphate and isopentenyl diphosphate to geranyl diphosphate, the key precursor of monoterpene biosynthesis, was purified from isolated oil glands of spearmint. Peptide fragments generated from the pure proteins of 28 and 37 kDa revealed amino acid sequences that matched two cDNA clones obtained by random screening of a peppermint-oil gland cDNA library. The deduced sequences of both proteins showed some similarity to existing prenyltransferases, and both contained a plastid-targeting sequence. Expression of each cDNA individually yielded no detectable prenyltransferase activity; however, coexpression of the two together produced functional geranyl diphosphate synthase. Antibodies raised against each protein were used to demonstrate that both subunits were required to produce catalytically active native and recombinant enzymes, thus confirming that geranyl diphosphate synthase is a heterodimer.
Figures


Similar articles
-
Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases.Plant Cell. 2004 Apr;16(4):977-92. doi: 10.1105/tpc.020156. Epub 2004 Mar 18. Plant Cell. 2004. PMID: 15031409 Free PMC article.
-
Protein design of geranyl diphosphate synthase. Structural features that define the product specificities of prenyltransferases.J Biochem. 1999 Sep;126(3):566-71. doi: 10.1093/oxfordjournals.jbchem.a022487. J Biochem. 1999. PMID: 10467173
-
Chrysanthemyl diphosphate synthase: isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium.Proc Natl Acad Sci U S A. 2001 Apr 10;98(8):4373-8. doi: 10.1073/pnas.071543598. Epub 2001 Apr 3. Proc Natl Acad Sci U S A. 2001. PMID: 11287653 Free PMC article.
-
Geranyl diphosphate synthase from Abies grandis: cDNA isolation, functional expression, and characterization.Arch Biochem Biophys. 2002 Sep 1;405(1):130-6. doi: 10.1016/s0003-9861(02)00335-1. Arch Biochem Biophys. 2002. PMID: 12176066
-
Monoterpene synthases from common sage (Salvia officinalis). cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase.J Biol Chem. 1998 Jun 12;273(24):14891-9. doi: 10.1074/jbc.273.24.14891. J Biol Chem. 1998. PMID: 9614092
Cited by
-
Genome-wide identification and characterization of novel genes involved in terpenoid biosynthesis in Salvia miltiorrhiza.J Exp Bot. 2012 Apr;63(7):2809-23. doi: 10.1093/jxb/err466. Epub 2012 Jan 30. J Exp Bot. 2012. PMID: 22291132 Free PMC article.
-
Monoterpene metabolism. Cloning, expression, and characterization of (-)-isopiperitenol/(-)-carveol dehydrogenase of peppermint and spearmint.Plant Physiol. 2005 Mar;137(3):863-72. doi: 10.1104/pp.104.053298. Epub 2005 Feb 25. Plant Physiol. 2005. PMID: 15734920 Free PMC article.
-
The genetic manipulation of medicinal and aromatic plants.Plant Cell Rep. 2007 Oct;26(10):1689-715. doi: 10.1007/s00299-007-0384-x. Epub 2007 Jul 3. Plant Cell Rep. 2007. PMID: 17609957 Review.
-
Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases.Plant Cell. 2004 Apr;16(4):977-92. doi: 10.1105/tpc.020156. Epub 2004 Mar 18. Plant Cell. 2004. PMID: 15031409 Free PMC article.
-
Structure and mechanism of an Arabidopsis medium/long-chain-length prenyl pyrophosphate synthase.Plant Physiol. 2011 Mar;155(3):1079-90. doi: 10.1104/pp.110.168799. Epub 2011 Jan 10. Plant Physiol. 2011. PMID: 21220764 Free PMC article.
References
-
- Poulter C D, Rilling H C. In: Biosynthesis of Isoprenoid Compounds. Porter J W, Spurgeon S L, editors. Vol. 1. New York: Wiley; 1981. pp. 161–224.
-
- Ogura K, Koyama T. Chem Rev. 1998;98:1263–1276. - PubMed
-
- Koyama T, Ogura K. In: Comprehensive Natural Products Chemistry. Cane D E, editor. Vol. 2. Oxford: Pergammon/Elsevier Science; 1999. pp. 69–96.
-
- Wang, K. & Ohnuma, S.-I. (1999) Trends Biochem. Sci., in press. - PubMed
-
- Tarshis L C, Yan M, Poulter C D, Sacchettini J C. Biochemistry. 1994;33:10871–10877. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

