Crystal structure of SQD1, an enzyme involved in the biosynthesis of the plant sulfolipid headgroup donor UDP-sulfoquinovose
- PMID: 10557279
- PMCID: PMC23906
- DOI: 10.1073/pnas.96.23.13097
Crystal structure of SQD1, an enzyme involved in the biosynthesis of the plant sulfolipid headgroup donor UDP-sulfoquinovose
Abstract
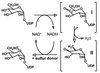
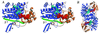
The SQD1 enzyme is believed to be involved in the biosynthesis of the sulfoquinovosyl headgroup of plant sulfolipids, catalyzing the transfer of SO(3)(-) to UDP-glucose. We have determined the structure of the complex of SQD1 from Arabidopsis thaliana with NAD(+) and the putative substrate UDP-glucose at 1.6-A resolution. Both bound ligands are completely buried within the binding cleft, along with an internal solvent cavity which is the likely binding site for the, as yet, unidentified sulfur-donor substrate. SQD1 is a member of the short-chain dehydrogenase/reductase (SDR) family of enzymes, and its structure shows a conservation of the SDR catalytic residues. Among several highly conserved catalytic residues, Thr-145 forms unusually short hydrogen bonds with both susceptible hydroxyls of UDP-glucose. A His side chain may also be catalytically important in the sulfonation.
Figures
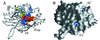
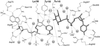

Similar articles
-
Prediction of the active-site structure and NAD(+) binding in SQD1, a protein essential for sulfolipid biosynthesis in Arabidopsis.Arch Biochem Biophys. 1999 Sep 1;369(1):30-41. doi: 10.1006/abbi.1999.1344. Arch Biochem Biophys. 1999. PMID: 10462438
-
Recombinant Arabidopsis SQD1 converts udp-glucose and sulfite to the sulfolipid head group precursor UDP-sulfoquinovose in vitro.J Biol Chem. 2001 Feb 9;276(6):3941-6. doi: 10.1074/jbc.M008200200. Epub 2000 Nov 9. J Biol Chem. 2001. PMID: 11073956
-
UDP-sulfoquinovose formation by Sulfolobus acidocaldarius.Extremophiles. 2015 Mar;19(2):451-67. doi: 10.1007/s00792-015-0730-9. Epub 2015 Jan 21. Extremophiles. 2015. PMID: 25605538 Free PMC article.
-
Biosynthesis and functions of the plant sulfolipid.Prog Lipid Res. 2011 Jul;50(3):234-9. doi: 10.1016/j.plipres.2011.02.003. Epub 2011 Mar 1. Prog Lipid Res. 2011. PMID: 21371504 Review.
-
UDP-hexose 4-epimerases: a view on structure, mechanism and substrate specificity.Carbohydr Res. 2015 Sep 23;414:8-14. doi: 10.1016/j.carres.2015.06.006. Epub 2015 Jun 21. Carbohydr Res. 2015. PMID: 26162744 Review.
Cited by
-
Does Sulfoquinovosyl Diacylglycerol Synthase OsSQD1 Affect the Composition of Lipids in Rice Phosphate-Deprived Root?Int J Mol Sci. 2022 Dec 21;24(1):114. doi: 10.3390/ijms24010114. Int J Mol Sci. 2022. PMID: 36613553 Free PMC article.
-
A cyanobacterial gene, sqdX, required for biosynthesis of the sulfolipid sulfoquinovosyldiacylglycerol.J Bacteriol. 2000 Jan;182(2):543-5. doi: 10.1128/JB.182.2.543-545.2000. J Bacteriol. 2000. PMID: 10629209 Free PMC article.
-
Arabidopsis disrupted in SQD2 encoding sulfolipid synthase is impaired in phosphate-limited growth.Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5732-7. doi: 10.1073/pnas.082696499. Proc Natl Acad Sci U S A. 2002. PMID: 11960029 Free PMC article.
-
Hub Genes in Stable QTLs Orchestrate the Accumulation of Cottonseed Oil in Upland Cotton via Catalyzing Key Steps of Lipid-Related Pathways.Int J Mol Sci. 2023 Nov 22;24(23):16595. doi: 10.3390/ijms242316595. Int J Mol Sci. 2023. PMID: 38068920 Free PMC article.
-
Medium- and short-chain dehydrogenase/reductase gene and protein families : the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes.Cell Mol Life Sci. 2008 Dec;65(24):3895-906. doi: 10.1007/s00018-008-8588-y. Cell Mol Life Sci. 2008. PMID: 19011750 Free PMC article. Review.
References
-
- Benning C. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:53–75. - PubMed
-
- Güler S, Seeliger A, Härtel H, Renger G, Benning C. J Biol Chem. 1996;271:7501–7507. - PubMed
-
- Gustafson K R, Cardellina J H, Fuller R W, Weislow O S, Kiser R F, Snader K M, Patterson G M, Boyd M R. J Natl Cancer Inst. 1989;81:1254–1258. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

