Mass spectrometric determination of dioxygen bond splitting in the "peroxy" intermediate of cytochrome c oxidase
- PMID: 10557282
- PMCID: PMC23909
- DOI: 10.1073/pnas.96.23.13114
Mass spectrometric determination of dioxygen bond splitting in the "peroxy" intermediate of cytochrome c oxidase
Abstract
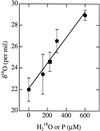
The "peroxy" intermediate (P form) of bovine cytochrome c oxidase was prepared by reaction of the two-electron reduced mixed-valence CO complex with (18)O(2) after photolytic removal of CO. The water present in the reaction mixture was recovered and analyzed for (18)O enrichment by mass spectrometry. It was found that approximately one oxygen atom ((18)O) per one equivalent of the P form was present in the bulk water. The data show that the oxygen-oxygen dioxygen bond is already broken in the P intermediate and that one oxygen atom can be readily released or exchanged with the oxygen of the solvent water.
Figures
Comment in
-
How oxygen is activated and reduced in respiration.Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):12971-3. doi: 10.1073/pnas.96.23.12971. Proc Natl Acad Sci U S A. 1999. PMID: 10557256 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

