BCR-ABL and v-SRC tyrosine kinase oncoproteins support normal erythroid development in erythropoietin receptor-deficient progenitor cells
- PMID: 10557295
- PMCID: PMC23922
- DOI: 10.1073/pnas.96.23.13186
BCR-ABL and v-SRC tyrosine kinase oncoproteins support normal erythroid development in erythropoietin receptor-deficient progenitor cells
Abstract
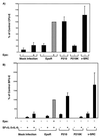
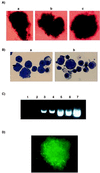
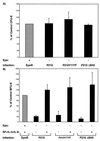
Erythropoietin (Epo)-independent differentiation of erythroid progenitors is a major characteristic of myeloproliferative disorders, including chronic myeloid leukemia. Epo receptor (EpoR) signaling is crucial for normal erythroid development, as evidenced by the properties of Epo(-/-) and EpoR(-/-) mice, which contain a normal number of fetal liver erythroid progenitors but die in utero from a severe anemia attributable to the absence of red cell maturation. Here we show that two constitutively active cytoplasmic protein tyrosine kinases, P210(BCR-ABL) and v-SRC, can functionally replace the EpoR and support full proliferation, differentiation, and maturation of fetal liver erythroid progenitors from EpoR(-/-) mice. These protein tyrosine kinases can also partially complement the myeloid growth factors IL-3, IL-6, and Steel factor, which are normally required in addition to Epo for erythroid development. Additionally, BCR-ABL mutants that lack residues necessary for transformation of fibroblasts or bone marrow cells can fully support normal erythroid development. These results demonstrate that activated tyrosine kinase oncoproteins implicated in tumorigenesis and human leukemia can functionally complement for cytokine receptor signaling pathways to support normal erythropoiesis in EpoR-deficient cells. Moreover, terminal differentiation of erythroid cells requires generic signals provided by activated protein tyrosine kinases and does not require a specific signal unique to a cytokine receptor.
Figures
Similar articles
-
Erythropoiesis in the absence of janus-kinase 2: BCR-ABL induces red cell formation in JAK2(-/-) hematopoietic progenitors.Blood. 2001 Nov 15;98(10):2948-57. doi: 10.1182/blood.v98.10.2948. Blood. 2001. PMID: 11698276
-
Expression of a constitutively active erythropoietin receptor in primary hematopoietic progenitors abrogates erythropoietin dependence and enhances erythroid colony-forming unit, erythroid burst-forming unit, and granulocyte/macrophage progenitor growth.Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):938-42. doi: 10.1073/pnas.90.3.938. Proc Natl Acad Sci U S A. 1993. PMID: 7679218 Free PMC article.
-
Interleukin-3 and p210 BCR/ABL activate both unique and overlapping pathways of signal transduction in a factor-dependent myeloid cell line.Exp Hematol. 1993 Oct;21(11):1460-6. Exp Hematol. 1993. PMID: 8405226
-
The role of tyrosine phosphorylation in proliferation and maturation of erythroid progenitor cells--signals emanating from the erythropoietin receptor.Eur J Biochem. 1997 Nov 1;249(3):637-47. doi: 10.1111/j.1432-1033.1997.t01-1-00637.x. Eur J Biochem. 1997. PMID: 9395308 Review.
-
Role of c-Kit and erythropoietin receptor in erythropoiesis.Crit Rev Oncol Hematol. 2005 Apr;54(1):63-75. doi: 10.1016/j.critrevonc.2004.11.005. Crit Rev Oncol Hematol. 2005. PMID: 15780908 Review.
Cited by
-
Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway.Blood. 2006 Feb 1;107(3):907-15. doi: 10.1182/blood-2005-06-2516. Epub 2005 Oct 4. Blood. 2006. PMID: 16204311 Free PMC article.
-
Molecular biology of chronic myeloid leukemia.Int J Hematol. 2001 Apr;73(3):308-22. doi: 10.1007/BF02981955. Int J Hematol. 2001. PMID: 11345196 Review.
-
Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor.Proc Natl Acad Sci U S A. 2003 May 27;100(11):6523-8. doi: 10.1073/pnas.0731871100. Epub 2003 May 15. Proc Natl Acad Sci U S A. 2003. PMID: 12750477 Free PMC article.
-
C/EBPalpha determines hematopoietic cell fate in multipotential progenitor cells by inhibiting erythroid differentiation and inducing myeloid differentiation.Blood. 2006 Jun 1;107(11):4308-16. doi: 10.1182/blood-2005-06-2216. Epub 2006 Feb 9. Blood. 2006. PMID: 16469877 Free PMC article.
-
SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system.Mol Biol Cell. 2003 Oct;14(10):3977-88. doi: 10.1091/mbc.e03-01-0001. Epub 2003 Jun 27. Mol Biol Cell. 2003. PMID: 14517312 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

