Increased thrombin responsiveness in platelets from mice lacking glycoprotein V
- PMID: 10557321
- PMCID: PMC23948
- DOI: 10.1073/pnas.96.23.13336
Increased thrombin responsiveness in platelets from mice lacking glycoprotein V
Abstract
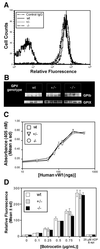
A role for glycoprotein (GP)V in platelet function has been proposed on the basis of observations that GP V is the major thrombin substrate on intact platelets cleaved during thrombin-induced platelet aggregation, and that GP V promotes GP Ib-IX surface expression in heterologous cells. We tested the hypotheses that GP V is involved in thrombin-induced platelet activation, in GP Ib-IX expression, and in other platelet responses by generating GP V null mice. Contrary to expectations, GP V -/- platelets were normal in size and expressed normal amounts of GP Ib-IX that was functional in von Willebrand factor binding, explaining why defects in GP V have not been observed in Bernard-Soulier syndrome, a bleeding disorder caused by a lack of functional GP Ib-IX-V. Moreover, in vitro analysis demonstrated that GP V -/- platelets were hyperresponsive to thrombin, resulting in increased fibrinogen binding and an increased aggregation response. Consistent with these findings, GP V -/- mice had a shorter bleeding time. These data support a role for GP V as a negative modulator of platelet activation. Furthermore, they suggest a new mechanism by which thrombin enhances platelet responsiveness independent of activation of the classical G-protein-coupled thrombin receptors.
Figures


References
-
- Baumgartner H R, Tschopp T B, Weiss H J. Thromb Haemostasis. 1978;39:782–783. - PubMed
-
- Clemetson K J, Clemetson J M. Semin Thromb Hemostasis. 1995;21:130–136. - PubMed
-
- Davies M J. Br Med Bull. 1994;50:789–802. - PubMed
-
- Jang Y, Lincoff A M, Plow E F, Topol E J. J Am Coll Cardiol. 1994;24:1591–1601. - PubMed
-
- Lopez J A, Andrews R K, Afshar-Kharghan V, Berndt M C. Blood. 1998;91:4397–4418. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

