Strychnine activates neuronal alpha7 nicotinic receptors after mutations in the leucine ring and transmitter binding site domains
- PMID: 10557336
- PMCID: PMC23963
- DOI: 10.1073/pnas.96.23.13421
Strychnine activates neuronal alpha7 nicotinic receptors after mutations in the leucine ring and transmitter binding site domains
Abstract
Recent work has shown that strychnine, the potent and selective antagonist of glycine receptors, is also an antagonist of nicotinic acetylcholine (AcCho) receptors including neuronal homomeric alpha7 receptors, and that mutating Leu-247 of the alpha7 nicotinic AcCho receptor-channel domain (L247Talpha7; mut1) converts some nicotinic antagonists into agonists. Therefore, a study was made of the effects of strychnine on Xenopus oocytes expressing the chick wild-type alpha7 or L247Talpha7 receptors. In these oocytes, strychnine itself did not elicit appreciable membrane currents but reduced the currents elicited by AcCho in a reversible and dose-dependent manner. In sharp contrast, in oocytes expressing L247Talpha(7) receptors with additional mutations at Cys-189 and Cys-190, in the extracellular N-terminal domain (L247T/C189-190Salpha7; mut2), micromolar concentrations of strychnine elicited inward currents that were reversibly inhibited by the nicotinic receptor blocker alpha-bungarotoxin. Single-channel recordings showed that strychnine gated mut2-channels with two conductance levels, 56 pS and 42 pS, and with kinetic properties similar to AcCho-activated channels. We conclude that strychnine is a modulator, as well as an activator, of some homomeric nicotinic alpha7 receptors. After injecting oocytes with mixtures of cDNAs encoding mut1 and mut2 subunits, the expressed hybrid receptors were activated by strychnine, similar to the mut2, and had a high affinity to AcCho like the mut1. A pentameric symmetrical model yields the striking conclusion that two identical alpha7 subunits may be sufficient to determine the functional properties of alpha7 receptors.
Figures
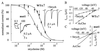

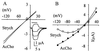



Similar articles
-
Effects of Zn2+ on wild and mutant neuronal alpha7 nicotinic receptors.Proc Natl Acad Sci U S A. 1998 Aug 18;95(17):10246-50. doi: 10.1073/pnas.95.17.10246. Proc Natl Acad Sci U S A. 1998. PMID: 9707632 Free PMC article.
-
Co-expression of the neuronal alpha7 and L247T alpha7 mutant subunits yields hybrid nicotinic receptors with properties of both wild-type alpha7 and alpha7 mutant homomeric receptors.Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1539-43. doi: 10.1073/pnas.94.4.1539. Proc Natl Acad Sci U S A. 1997. PMID: 9037089 Free PMC article.
-
Neuronal nicotinic threonine-for-leucine 247 alpha7 mutant receptors show different gating kinetics when activated by acetylcholine or by the noncompetitive agonist 5-hydroxytryptamine.Proc Natl Acad Sci U S A. 1997 Sep 2;94(18):9915-9. doi: 10.1073/pnas.94.18.9915. Proc Natl Acad Sci U S A. 1997. PMID: 9275226 Free PMC article.
-
Nicotinic receptors containing the alpha7 subunit: a model for rational drug design.Curr Med Chem. 2008;15(28):2921-32. doi: 10.2174/092986708786848703. Curr Med Chem. 2008. PMID: 19075644 Free PMC article. Review.
-
Use of concatemers of ligand-gated ion channel subunits to study mechanisms of steroid potentiation.Anesthesiology. 2011 Dec;115(6):1328-37. doi: 10.1097/ALN.0b013e318233046a. Anesthesiology. 2011. PMID: 21926904 Free PMC article. Review.
Cited by
-
Tightening of the ATP-binding sites induces the opening of P2X receptor channels.EMBO J. 2012 May 2;31(9):2134-43. doi: 10.1038/emboj.2012.75. Epub 2012 Mar 30. EMBO J. 2012. PMID: 22473210 Free PMC article.
-
Neuronally produced betaine acts via a ligand-gated ion channel to control behavioral states.Proc Natl Acad Sci U S A. 2022 Nov 29;119(48):e2201783119. doi: 10.1073/pnas.2201783119. Epub 2022 Nov 21. Proc Natl Acad Sci U S A. 2022. PMID: 36413500 Free PMC article.
-
Evidence for a centrally located gate in the pore of a serotonin-gated ion channel.J Neurosci. 2002 Mar 1;22(5):1629-39. doi: 10.1523/JNEUROSCI.22-05-01629.2002. J Neurosci. 2002. PMID: 11880493 Free PMC article.
-
Amyloid beta(1-42) peptide alters the gating of human and mouse alpha-bungarotoxin-sensitive nicotinic receptors.J Physiol. 2003 Feb 15;547(Pt 1):147-57. doi: 10.1113/jphysiol.2002.035436. Epub 2003 Jan 17. J Physiol. 2003. PMID: 12562926 Free PMC article.
-
A Novel and Functionally Diverse Class of Acetylcholine-Gated Ion Channels.J Neurosci. 2023 Feb 15;43(7):1111-1124. doi: 10.1523/JNEUROSCI.1516-22.2022. Epub 2023 Jan 5. J Neurosci. 2023. PMID: 36604172 Free PMC article.
References
-
- Matsubayashi H, Alkondon M, Pereira E F, Swanson K L, Albuquerque E X. J Pharmacol Exp Ther. 1998;284:904–913. - PubMed
-
- Anand R, Peng X, Lindstrom J. FEBS Lett. 1993;327:241–246. - PubMed
-
- Anand R, Peng X, Ballesta J, Lindstrom J. Mol Pharmacol. 1993;44:1046–1050. - PubMed
-
- Gerzanich V, Anand R, Lindstrom J. Mol Pharmacol. 1994;45:212–220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

