Developmental regulation of spine motility in the mammalian central nervous system
- PMID: 10557339
- PMCID: PMC23966
- DOI: 10.1073/pnas.96.23.13438
Developmental regulation of spine motility in the mammalian central nervous system
Abstract
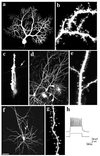
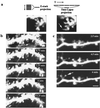
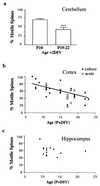
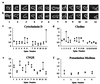
The function of dendritic spines, postsynaptic sites of excitatory input in the mammalian central nervous system (CNS), is still not well understood. Although changes in spine morphology may mediate synaptic plasticity, the extent of basal spine motility and its regulation and function remains controversial. We investigated spine motility in three principal neurons of the mouse CNS: cerebellar Purkinje cells, and cortical and hippocampal pyramidal neurons. Motility was assayed with time-lapse imaging by using two-photon microscopy of green fluorescent protein-labeled neurons in acute and cultured slices. In all three cell types, dendritic protrusions (filopodia and spines) were highly dynamic, exhibiting a diversity of morphological rearrangements over short (<1-min) time courses. The incidence of spine motility declined during postnatal maturation, but dynamic changes were still apparent in many spines in late-postnatal neurons. Although blockade or induction of neuronal activity did not affect spine motility, disruption of actin polymerization did. We hypothesize that this basal motility of dendritic protrusions is intrinsic to the neuron and underlies the heightened plasticity found in developing CNS.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

