Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein
- PMID: 10557341
- PMCID: PMC23968
- DOI: 10.1073/pnas.96.23.13450
Axon pathology in Parkinson's disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein
Abstract
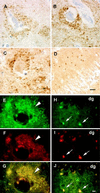
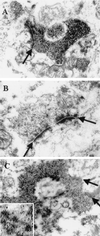
Pathogenic alpha-synuclein (alphaS) gene mutations occur in rare familial Parkinson's disease (PD) kindreds, and wild-type alphaS is a major component of Lewy bodies (LBs) in sporadic PD, dementia with LBs (DLB), and the LB variant of Alzheimer's disease, but beta-synuclein (betaS) and gamma-synuclein (gammaS) have not yet been implicated in neurological disorders. Here we show that in PD and DLB, but not normal brains, antibodies to alphaS and betaS reveal novel presynaptic axon terminal pathology in the hippocampal dentate, hilar, and CA2/3 regions, whereas antibodies to gammaS detect previously unrecognized axonal spheroid-like lesions in the hippocampal dentate molecular layer. The aggregation of other synaptic proteins and synaptic vesicle-like structures in the alphaS- and betaS-labeled hilar dystrophic neurites suggests that synaptic dysfunction may result from these lesions. Our findings broaden the concept of neurodegenerative "synucleinopathies" by implicating betaS and gammaS, in addition to alphaS, in the onset/progression of PD and DLB.
Figures



References
-
- Jakes R, Spillantini M G, Goedert M. FEBS Lett. 1994;345:27–32. - PubMed
-
- Tobe T, Nakajo S, Tanaka A, Mitoya A, Omata K, Nakaya K, Tomita M, Nakmura Y. J Neurochem. 1992;59:1624–1629. - PubMed
-
- Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. Eur J Biochem. 1993;217:1057–1063. - PubMed
-
- Ji H, Liu Y E, Jia T, Wang M, Liu J, Xiao G, Joseph B K, Rosen C, Shi Y E. Cancer Res. 1997;57:759–764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

