Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+
- PMID: 10557359
- PMCID: PMC23986
- DOI: 10.1073/pnas.96.23.13554
Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+
Abstract
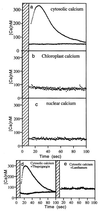
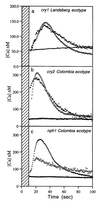
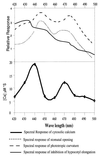
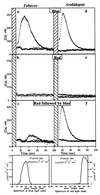
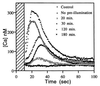
Blue light regulates plant growth and development, and three photoreceptors, CRY1, CRY2, and NPH1, have been identified. The transduction pathways of these receptors are poorly understood. Transgenic plants containing aequorin have been used to dissect the involvement of these three receptors in the regulation of intracellular Ca2+. Pulses of blue light induce cytosolic Ca2+ transients lasting about 80 s in Arabidopsis and tobacco seedlings. Use of organelle-targeted aequorins shows that Ca2+ increases are limited to the cytoplasm. Blue light treatment of cry1, cry2, and nph1 mutants showed that NPH1, which regulates phototropism, is largely responsible for the Ca2+ transient. The spectral response of the Ca2+ transient is similar to that of phototropism, supporting NPH1 involvement. Furthermore, known interactions between red and blue light and between successive blue light pulses on phototropic sensitivity are mirrored in the blue light control of cytosolic Ca2+ in these seedlings. Our observations raise the possibility that physiological responses regulated by NPH1, such as phototropism, may be transduced through cytosolic Ca2+.
Figures
References
-
- Ahmad M, Cashmore A R. Nature (London) 1993;366:162–166. - PubMed
-
- Ahmad M, Lin C T, Cashmore A R. Plant J. 1995;8:653–658. - PubMed
-
- Jackson J A, Jenkins G I. Planta. 1995;197:233–239. - PubMed
-
- Lin C T, Robertson D E, Ahmad M, Raibekas A A, Jorns M S, Dutton P L, Cashmore A R. Science. 1995;269:968–970. - PubMed
-
- Malhotra K, Kim S-T, Batschauer A, Dawut L, Sancar A. Biochemistry. 1995;34:6892–6899. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

