Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis
- PMID: 10559153
- PMCID: PMC94162
- DOI: 10.1128/JB.181.22.6889-6897.1999
Catabolite regulation of the pta gene as part of carbon flow pathways in Bacillus subtilis
Abstract
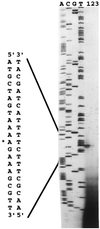
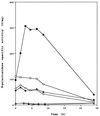
In Bacillus subtilis, the products of the pta and ackA genes, phosphotransacetylase and acetate kinase, play a crucial role in the production of acetate, one of the most abundant by-products of carbon metabolism in this gram-positive bacterium. Although these two enzymes are part of the same pathway, only mutants with inactivated ackA did not grow in the presence of glucose. Inactivation of pta had only a weak inhibitory effect on growth. In contrast to pta and ackA in Escherichia coli, the corresponding B. subtilis genes are not cotranscribed. Expression of the pta gene was increased in the presence of glucose, as has been reported for ackA. The effects of the predicted cis-acting catabolite response element (CRE) located upstream from the promoter and of the trans-acting proteins CcpA, HPr, Crh, and HPr kinase on the catabolite regulation of pta were investigated. As for ackA, glucose activation was abolished in ccpA and hprK mutants and in the ptsH1 crh double mutant. Footprinting experiments demonstrated an interaction between CcpA and the pta CRE sequence, which is almost identical to the proposed CRE consensus sequence. This interaction occurs only in the presence of Ser-46-phosphorylated HPr (HPrSer-P) or Ser-46-phosphorylated Crh (CrhSer-P) and fructose-1,6-bisphosphate (FBP). In addition to CcpA, carbon catabolite activation of the pta gene therefore requires at least two other cofactors, FBP and either HPr or Crh, phosphorylated at Ser-46 by the ATP-dependent Hpr kinase.
Figures



References
-
- Brown T D K, Jones-Mortimer M C, Kornberg H L. The enzymatic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977;102:327–336. - PubMed
-
- Deutscher J, Kuster E, Bergstedt U, Charrier V, Hillen W. Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in gram-positive bacteria. Mol Microbiol. 1995;15:1049–1053. - PubMed
-
- Deutscher J, Reizer J, Fischer C, Galinier A, Saier M H, Jr, Steinmetz M. Loss of protein kinase-catalyzed phosphorylation of HPr, a phosphocarrier protein of the phosphotransferase system, by mutation of the ptsH gene confers catabolite repression resistance to several catabolic genes of Bacillus subtilis. J Bacteriol. 1994;176:3336–3344. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

