Phenotypic consequences resulting from a methionine-to-valine substitution at position 48 in the HPr protein of Streptococcus salivarius
- PMID: 10559156
- PMCID: PMC94165
- DOI: 10.1128/JB.181.22.6914-6921.1999
Phenotypic consequences resulting from a methionine-to-valine substitution at position 48 in the HPr protein of Streptococcus salivarius
Abstract
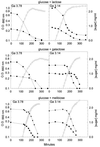
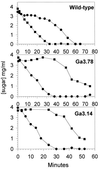
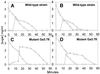
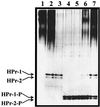
In gram-positive bacteria, the HPr protein of the phosphoenolpyruvate:sugar phosphotransferase system (PTS) can be phosphorylated on a histidine residue at position 15 (His(15)) by enzyme I (EI) of the PTS and on a serine residue at position 46 (Ser(46)) by an ATP-dependent protein kinase (His approximately P and Ser-P, respectively). We have isolated from Streptococcus salivarius ATCC 25975, by independent selection from separate cultures, two spontaneous mutants (Ga3.78 and Ga3.14) that possess a missense mutation in ptsH (the gene encoding HPr) replacing the methionine at position 48 by a valine. The mutation did not prevent the phosphorylation of HPr at His(15) by EI nor the phosphorylation at Ser(46) by the ATP-dependent HPr kinase. The levels of HPr(Ser-P) in glucose-grown cells of the parental and mutant Ga3.78 were virtually the same. However, mutant cells growing on glucose produced two- to threefold less HPr(Ser-P)(His approximately P) than the wild-type strain, while the levels of free HPr and HPr(His approximately P) were increased 18- and 3-fold, respectively. The mutants grew as well as the wild-type strain on PTS sugars (glucose, fructose, and mannose) and on the non-PTS sugars lactose and melibiose. However, the growth rate of both mutants on galactose, also a non-PTS sugar, decreased rapidly with time. The M48V substitution had only a minor effect on the repression of alpha-galactosidase, beta-galactosidase, and galactokinase by glucose, but this mutation abolished diauxie by rendering cells unable to prevent the catabolism of a non-PTS sugar (lactose, galactose, and melibiose) when glucose was available. The results suggested that the capacity of the wild-type cells to preferentially metabolize glucose over non-PTS sugars resulted mainly from inhibition of the catabolism of these secondary energy sources via a HPr-dependent mechanism. This mechanism was activated following glucose but not lactose metabolism, and it did not involve HPr(Ser-P) as the only regulatory molecule.
Figures


Similar articles
-
Diversity of Streptococcus salivarius ptsH mutants that can be isolated in the presence of 2-deoxyglucose and galactose and characterization of two mutants synthesizing reduced levels of HPr, a phosphocarrier of the phosphoenolpyruvate:sugar phosphotransferase system.J Bacteriol. 2001 Sep;183(17):5145-54. doi: 10.1128/JB.183.17.5145-5154.2001. J Bacteriol. 2001. PMID: 11489868 Free PMC article.
-
Phosphorylation of Streptococcus salivarius lactose permease (LacS) by HPr(His ~ P) and HPr(Ser-P)(His ~ P) and effects on growth.J Bacteriol. 2003 Dec;185(23):6764-72. doi: 10.1128/JB.185.23.6764-6772.2003. J Bacteriol. 2003. PMID: 14617640 Free PMC article.
-
Replacement of isoleucine-47 by threonine in the HPr protein of Streptococcus salivarius abrogates the preferential metabolism of glucose and fructose over lactose and melibiose but does not prevent the phosphorylation of HPr on serine-46.Mol Microbiol. 1997 Aug;25(4):695-705. doi: 10.1046/j.1365-2958.1997.4981870.x. Mol Microbiol. 1997. PMID: 9379899
-
Catabolite repression and inducer control in Gram-positive bacteria.Microbiology (Reading). 1996 Feb;142 ( Pt 2):217-230. doi: 10.1099/13500872-142-2-217. Microbiology (Reading). 1996. PMID: 8932696 Review.
-
The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism.FEMS Microbiol Rev. 1997 Feb;19(3):187-207. doi: 10.1111/j.1574-6976.1997.tb00297.x. FEMS Microbiol Rev. 1997. PMID: 9050218 Review.
Cited by
-
Diversity of Streptococcus salivarius ptsH mutants that can be isolated in the presence of 2-deoxyglucose and galactose and characterization of two mutants synthesizing reduced levels of HPr, a phosphocarrier of the phosphoenolpyruvate:sugar phosphotransferase system.J Bacteriol. 2001 Sep;183(17):5145-54. doi: 10.1128/JB.183.17.5145-5154.2001. J Bacteriol. 2001. PMID: 11489868 Free PMC article.
-
Phosphorylation of Streptococcus salivarius lactose permease (LacS) by HPr(His ~ P) and HPr(Ser-P)(His ~ P) and effects on growth.J Bacteriol. 2003 Dec;185(23):6764-72. doi: 10.1128/JB.185.23.6764-6772.2003. J Bacteriol. 2003. PMID: 14617640 Free PMC article.
-
The doubly phosphorylated form of HPr, HPr(Ser~P)(His-P), is abundant in exponentially growing cells of Streptococcus thermophilus and phosphorylates the lactose transporter LacS as efficiently as HPr(His~P).Appl Environ Microbiol. 2005 Mar;71(3):1364-72. doi: 10.1128/AEM.71.3.1364-1372.2005. Appl Environ Microbiol. 2005. PMID: 15746339 Free PMC article.
-
How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria.Microbiol Mol Biol Rev. 2006 Dec;70(4):939-1031. doi: 10.1128/MMBR.00024-06. Microbiol Mol Biol Rev. 2006. PMID: 17158705 Free PMC article. Review.
-
Sinorhizobium meliloti mutants lacking phosphotransferase system enzyme HPr or EIIA are altered in diverse processes, including carbon metabolism, cobalt requirements, and succinoglycan production.J Bacteriol. 2008 Apr;190(8):2947-56. doi: 10.1128/JB.01917-07. Epub 2008 Feb 15. J Bacteriol. 2008. PMID: 18281401 Free PMC article.
References
-
- Avigad G, Amaval D, Ascensio C, Horecker B L. The d-galactose oxydase of Polyporus circinatus. J Biol Chem. 1962;237:2736–2743. - PubMed
-
- Bourassa S, Gauthier L, Giguère R, Vadeboncoeur C. A IIIMan protein is involved in the transport of glucose, mannose, and fructose by oral streptococci. Oral Microbiol Immunol. 1990;5:288–297. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Deutscher J, Engelmann R. Purification and characterization of an ATP-dependent protein kinase from Streptococcus faecalis. FEMS Microbiol Lett. 1984;23:157–162.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

