The respiratory system and diazotrophic activity of Acetobacter diazotrophicus PAL5
- PMID: 10559164
- PMCID: PMC94173
- DOI: 10.1128/JB.181.22.6987-6995.1999
The respiratory system and diazotrophic activity of Acetobacter diazotrophicus PAL5
Abstract
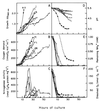
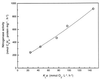
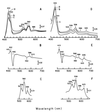
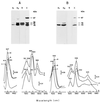
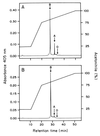
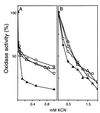
The characteristics of the respiratory system of Acetobacter diazotrophicus PAL5 were investigated. Increasing aeration (from 0.5 to 4.0 liters of air min(-1) liter of medium(-1)) had a strong positive effect on growth and on the diazotrophic activity of cultures. Cells obtained from well-aerated and diazotrophically active cultures possessed a highly active, membrane-bound electron transport system with dehydrogenases for NADH, glucose, and acetaldehyde as the main electron donors. Ethanol, succinate, and gluconate were also oxidized but to only a minor extent. Terminal cytochrome c oxidase-type activity was poor as measured by reduced N, N,N,N'-tetramethyl-p-phenylenediamine, but quinol oxidase-type activity, as measured by 2,3,5,6-tetrachloro-1,4-benzenediol, was high. Spectral and high-pressure liquid chromatography analysis of membranes revealed the presence of cytochrome ba as a putative oxidase in cells obtained from diazotrophically active cultures. Cells were also rich in c-type cytochromes; four bands of high molecular mass (i.e., 67, 56, 52, and 45 kDa) were revealed by a peroxidase activity stain in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. KCN inhibition curves of respiratory oxidase activities were biphasic, with a highly resistant component. Treatment of membranes with 0.2% Triton X-100 solubilized c-type cytochromes and resulted in a preparation that was significantly more sensitive to cyanide. Repression of diazotrophic activity in well-aerated cultures by 40 mM (NH(4))(2)SO(4) caused a significant decrease of the respiratory activities. It is noteworthy that the levels of glucose dehydrogenase and putative oxidase ba decreased 6. 8- and 10-fold, respectively. In these cells, a bd-type cytochrome seems to be the major terminal oxidase. Thus, it would seem that glucose dehydrogenase and cytochrome ba are key components of the respiratory system of A. diazotrophicus during aerobic diazotrophy.
Figures

Similar articles
-
Respiratory system of Gluconacetobacter diazotrophicus PAL5. Evidence for a cyanide-sensitive cytochrome bb and cyanide-resistant cytochrome ba quinol oxidases.Biochim Biophys Acta. 2006 Dec;1757(12):1614-22. doi: 10.1016/j.bbabio.2006.06.013. Epub 2006 Jul 8. Biochim Biophys Acta. 2006. PMID: 16934215
-
The carbon source influences the energetic efficiency of the respiratory chain of N2-fixing Acetobacter diazotrophicus.Appl Microbiol Biotechnol. 2000 Oct;54(4):564-9. doi: 10.1007/s002530000425. Appl Microbiol Biotechnol. 2000. PMID: 11092633
-
Transient accumulation of heme O (cytochrome o) in the cytoplasmic membrane of semi-anaerobic Anacystis nidulans. Evidence for oxygenase-catalyzed heme O/A transformation.J Biol Chem. 1995 Nov 17;270(46):27937-41. doi: 10.1074/jbc.270.46.27937. J Biol Chem. 1995. PMID: 7499269
-
Change of the terminal oxidase from cytochrome a1 in shaking cultures to cytochrome o in static cultures of Acetobacter aceti.J Bacteriol. 1992 Jan;174(1):122-9. doi: 10.1128/jb.174.1.122-129.1992. J Bacteriol. 1992. PMID: 1729204 Free PMC article.
-
Methanol and ethanol oxidase respiratory chains of the methylotrophic acetic acid bacterium, Acetobacter methanolicus.J Biochem. 1992 Jun;111(6):739-47. doi: 10.1093/oxfordjournals.jbchem.a123829. J Biochem. 1992. PMID: 1323563
Cited by
-
CowN sustains nitrogenase turnover in the presence of the inhibitor carbon monoxide.J Biol Chem. 2021 Jan-Jun;296:100501. doi: 10.1016/j.jbc.2021.100501. Epub 2021 Mar 2. J Biol Chem. 2021. PMID: 33667548 Free PMC article.
-
Evidence for conformational protection of nitrogenase against oxygen in Gluconacetobacter diazotrophicus by a putative FeSII protein.J Bacteriol. 2002 Oct;184(20):5805-9. doi: 10.1128/JB.184.20.5805-5809.2002. J Bacteriol. 2002. PMID: 12270840 Free PMC article.
-
Physiological uncoupling of mitochondrial oxidative phosphorylation. Studies in different yeast species.J Bioenerg Biomembr. 2011 Jun;43(3):323-31. doi: 10.1007/s10863-011-9356-5. J Bioenerg Biomembr. 2011. PMID: 21556887
-
Antioxidant pathways are up-regulated during biological nitrogen fixation to prevent ROS-induced nitrogenase inhibition in Gluconacetobacter diazotrophicus.Arch Microbiol. 2010 Oct;192(10):835-41. doi: 10.1007/s00203-010-0609-1. Epub 2010 Aug 10. Arch Microbiol. 2010. PMID: 20697694 Free PMC article.
-
The oxidative fermentation of ethanol in Gluconacetobacter diazotrophicus is a two-step pathway catalyzed by a single enzyme: alcohol-aldehyde Dehydrogenase (ADHa).Int J Mol Sci. 2015 Jan 7;16(1):1293-311. doi: 10.3390/ijms16011293. Int J Mol Sci. 2015. PMID: 25574602 Free PMC article.
References
-
- Alvarez B, Martínez-Drets G. Metabolic characterization of Acetobacter diazotrophicus. Can J Microbiol. 1995;41:918–924.
-
- Ameyama M, Adachi O. Alcohol dehydrogenase from acetic acid bacteria, membrane-bound. Methods Enzymol. 1982;89:450–457.
-
- Ameyama M, Adachi O. Aldehyde dehydrogenase from acetic acid bacteria, membrane-bound. Methods Enzymol. 1982;89:491–497.
-
- Ameyama M, Matsushita K, Shinagawa E, Adachi O. Sugar-oxidizing respiratory chain of Gluconobacter suboxydans. Evidence for a branched respiratory chain and characterization of respiratory chain-linked cytochromes. Agric Biol Chem. 1987;51:2943–2950.
-
- Attwood M M, van Dijken J P, Pronk J T. Glucose metabolism and gluconic acid production by Acetobacter diazotrophicus. J Ferment Bioeng. 1991;72:101–105.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

