Characterization of the essential transport function of the AIDA-I autotransporter and evidence supporting structural predictions
- PMID: 10559167
- PMCID: PMC94176
- DOI: 10.1128/JB.181.22.7014-7020.1999
Characterization of the essential transport function of the AIDA-I autotransporter and evidence supporting structural predictions
Abstract
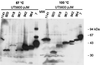
The current model for autodisplay suggests a mechanism that allows a passenger protein to be translocated across the outer membrane by coordinate action of a C-terminal beta-barrel and its preceding linking region. The passenger protein, linker, and beta-barrel are together termed the autotransporter, while the linker and beta-barrel are here referred to as the translocation unit (TU). We characterized the minimal TU necessary for autodisplay with the adhesin-involved-in-diffuse-adherence (AIDA-I) autotransporter. The assumed beta-barrel structure at the C terminus of the AIDA-I autotransporter was studied by constructing a set of seven AIDA-I-cholera toxin B subunit fusion proteins containing various portions of AIDA-I. Surface exposure of the cholera toxin B moiety was assessed by dot blot experiments and trypsin accessibility of the chimeric proteins expressed in Escherichia coli JK321 or UT5600. Export of cholera toxin B strictly depended on a complete predicted beta-barrel region. The absolute necessity for export of a linking region and its influence on expression as an integral part of the TU was also demonstrated. The different electrophoretic mobilities of native and denatured chimeras indicated that the proposed beta-barrel resides within the C-terminal 312 amino acids of AIDA-I. Together these data provide evidence for the predicted beta-barrel structure and support our formerly proposed model of membrane topology of the AIDA-I autotransporter.
Figures

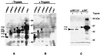
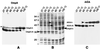

Similar articles
-
The autodisplay story, from discovery to biotechnical and biomedical applications.Microbiol Mol Biol Rev. 2007 Dec;71(4):600-19. doi: 10.1128/MMBR.00011-07. Microbiol Mol Biol Rev. 2007. PMID: 18063719 Free PMC article. Review.
-
Autodisplay: one-component system for efficient surface display and release of soluble recombinant proteins from Escherichia coli.J Bacteriol. 1997 Feb;179(3):794-804. doi: 10.1128/jb.179.3.794-804.1997. J Bacteriol. 1997. PMID: 9006035 Free PMC article.
-
Crystal structure of the transport unit of the autotransporter adhesin involved in diffuse adherence from Escherichia coli.J Struct Biol. 2014 Jul;187(1):20-29. doi: 10.1016/j.jsb.2014.05.003. Epub 2014 May 16. J Struct Biol. 2014. PMID: 24841284
-
Arrangement of the translocator of the autotransporter adhesin involved in diffuse adherence on the bacterial surface.Infect Immun. 2005 Jul;73(7):3851-9. doi: 10.1128/IAI.73.7.3851-3859.2005. Infect Immun. 2005. PMID: 15972470 Free PMC article.
-
Self-associating autotransporters, SAATs: functional and structural similarities.Int J Med Microbiol. 2006 Aug;296(4-5):187-95. doi: 10.1016/j.ijmm.2005.10.002. Int J Med Microbiol. 2006. PMID: 16600681 Review.
Cited by
-
Role of the alpha-helical linker of the C-terminal translocator in the biogenesis of the serine protease subfamily of autotransporters.Infect Immun. 2006 Sep;74(9):4961-9. doi: 10.1128/IAI.00103-06. Infect Immun. 2006. PMID: 16926387 Free PMC article.
-
Identification of secretion determinants of the Bordetella pertussis BrkA autotransporter.J Bacteriol. 2003 Jan;185(2):489-95. doi: 10.1128/JB.185.2.489-495.2003. J Bacteriol. 2003. PMID: 12511495 Free PMC article.
-
The Moraxella catarrhalis autotransporter McaP is a conserved surface protein that mediates adherence to human epithelial cells through its N-terminal passenger domain.Infect Immun. 2007 Jan;75(1):314-24. doi: 10.1128/IAI.01330-06. Epub 2006 Nov 6. Infect Immun. 2007. PMID: 17088358 Free PMC article.
-
The autodisplay story, from discovery to biotechnical and biomedical applications.Microbiol Mol Biol Rev. 2007 Dec;71(4):600-19. doi: 10.1128/MMBR.00011-07. Microbiol Mol Biol Rev. 2007. PMID: 18063719 Free PMC article. Review.
-
Esterase autodisplay: enzyme engineering and whole-cell activity determination in microplates with pH sensors.Appl Environ Microbiol. 2008 Aug;74(15):4782-91. doi: 10.1128/AEM.01575-07. Epub 2008 May 30. Appl Environ Microbiol. 2008. PMID: 18515492 Free PMC article.
References
-
- Anderson D M, Schneewind O. A mRNA signal for the type III secretion of Yop proteins by Yersinia enterocolitica. Science. 1997;278:1140–1143. - PubMed
-
- Baneyx F, Ayling A, Palumbo T, Thomas D, Georgiou G. Optimization of growth conditions for the production of proteolytically-sensitive proteins in the periplasmic space of Escherichia coli. Appl Microbiol Biotechnol. 1991;36:14–20. - PubMed
-
- Baneyx F, Georgiou G. Degradation of secreted proteins in Escherichia coli. Ann NY Acad Sci. 1992;665:301–308. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

