Functional regions of the Bacillus subtilis spore coat morphogenetic protein CotE
- PMID: 10559171
- PMCID: PMC94180
- DOI: 10.1128/JB.181.22.7043-7051.1999
Functional regions of the Bacillus subtilis spore coat morphogenetic protein CotE
Abstract
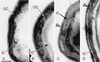
The Bacillus subtilis spore is encased in a resilient, multilayered proteinaceous shell, called the coat, that protects it from the environment. A 181-amino-acid coat protein called CotE assembles into the coat early in spore formation and plays a morphogenetic role in the assembly of the coat's outer layer. We have used a series of mutant alleles of cotE to identify regions involved in outer coat protein assembly. We found that the insertion of a 10-amino-acid epitope, between amino acids 178 and 179 of CotE, reduced or prevented the assembly of several spore coat proteins, including, most likely, CotG and CotB. The removal of 9 or 23 of the C-terminal-most amino acids resulted in an unusually thin outer coat from which a larger set of spore proteins was missing. In contrast, the removal of 37 amino acids from the C terminus, as well as other alterations between amino acids 4 and 160, resulted in the absence of a detectable outer coat but did not prevent localization of CotE to the forespore. These results indicate that changes in the C-terminal 23 amino acids of CotE and in the remainder of the protein have different consequences for outer coat protein assembly.
Figures



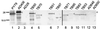

References
-
- Abe A, Koide H, Kohno T, Watabe K. A Bacillus subtilis spore coat polypeptide gene, cotS. Microbiology. 1995;141:1433–1442. - PubMed
-
- Cutting S M, Vander Horn P B. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1990.
-
- Donovan W, Zheng L, Sandman K, Losick R. Genes encoding spore coat polypeptides from Bacillus subtilis. J Mol Biol. 1987;196:1–10. - PubMed
-
- Driks, A. Unpublished observations.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

