Role of the epstein-barr virus RTA protein in activation of distinct classes of viral lytic cycle genes
- PMID: 10559298
- PMCID: PMC113035
- DOI: 10.1128/JVI.73.12.9858-9866.1999
Role of the epstein-barr virus RTA protein in activation of distinct classes of viral lytic cycle genes
Abstract
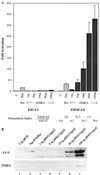
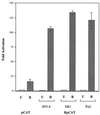
Initiation of the Epstein-Barr virus (EBV) lytic cycle is controlled by two immediate-early genes, BZLF1 and BRLF1. In certain epithelial and B-cell lines, their protein products, ZEBRA and Rta, stimulate their own expression, reciprocally stimulate each other's expression, and activate downstream viral targets. It has been difficult to examine the individual roles of these two transactivators in EBV-infected lymphocytes, as they are expressed simultaneously upon induction of the lytic cycle. Here we show that the Burkitt lymphoma cell line Raji represents an experimental system that allows the study of Rta's role in the lytic cycle of EBV in the absence and presence of ZEBRA. When expressed in Raji cells, exogenous Rta does not activate endogenous BZLF1 expression, yet Rta remains competent to transactivate certain downstream viral targets. Some genes, such as BaRF1, BMLF1, and a late gene, BLRF2, are maximally activated by Rta itself in the absence of detectable ZEBRA. The use of the Z(S186A) mutant form of ZEBRA, whose transactivation function is manifest only by coexpression of Rta, allows identification of a second class of lytic cycle genes, such as BMRF1 and BHRF1, that are activated in synergy by Rta and ZEBRA. It has already been documented that of the two activators, only ZEBRA stimulates the BRLF1 gene in Raji cells. Thus, there is a third class of viral genes activated by ZEBRA but not Rta. Moreover, ZEBRA exhibits an inhibitory effect on Rta's capacity to stimulate the late gene, BLRF2. Consequently ZEBRA may function to repress Rta's potential to activate some late genes. Raji cells thus allow delineation of the combinatorial roles of Rta and ZEBRA in control of several distinct classes of lytic cycle genes.
Figures





Similar articles
-
Amino acid substitutions reveal distinct functions of serine 186 of the ZEBRA protein in activation of early lytic cycle genes and synergy with the Epstein-Barr virus R transactivator.J Virol. 1999 Jun;73(6):4543-51. doi: 10.1128/JVI.73.6.4543-4551.1999. J Virol. 1999. PMID: 10233912 Free PMC article.
-
Phosphorylation of Epstein-Barr virus ZEBRA protein at its casein kinase 2 sites mediates its ability to repress activation of a viral lytic cycle late gene by Rta.J Virol. 2004 Jul;78(14):7634-44. doi: 10.1128/JVI.78.14.7634-7644.2004. J Virol. 2004. PMID: 15220438 Free PMC article.
-
The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes.J Virol. 1998 Oct;72(10):7978-84. doi: 10.1128/JVI.72.10.7978-7984.1998. J Virol. 1998. PMID: 9733836 Free PMC article.
-
Lytic cycle switches of oncogenic human gammaherpesviruses.Adv Cancer Res. 2007;97:81-109. doi: 10.1016/S0065-230X(06)97004-3. Adv Cancer Res. 2007. PMID: 17419942 Review.
-
Oncogenic Properties of the EBV ZEBRA Protein.Cancers (Basel). 2020 Jun 5;12(6):1479. doi: 10.3390/cancers12061479. Cancers (Basel). 2020. PMID: 32517128 Free PMC article. Review.
Cited by
-
Rta is the principal activator of Epstein-Barr virus epithelial lytic transcription.PLoS Pathog. 2022 Sep 29;18(9):e1010886. doi: 10.1371/journal.ppat.1010886. eCollection 2022 Sep. PLoS Pathog. 2022. PMID: 36174106 Free PMC article.
-
Epstein-Barr virus BRLF1 induces genomic instability and progressive malignancy in nasopharyngeal carcinoma cells.Oncotarget. 2017 Sep 5;8(45):78948-78964. doi: 10.18632/oncotarget.20695. eCollection 2017 Oct 3. Oncotarget. 2017. PMID: 29108278 Free PMC article.
-
Viral genome methylation differentially affects the ability of BZLF1 versus BRLF1 to activate Epstein-Barr virus lytic gene expression and viral replication.J Virol. 2013 Jan;87(2):935-50. doi: 10.1128/JVI.01790-12. Epub 2012 Nov 7. J Virol. 2013. PMID: 23135711 Free PMC article.
-
Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters.J Virol. 2001 Feb;75(4):1798-807. doi: 10.1128/JVI.75.4.1798-1807.2001. J Virol. 2001. PMID: 11160678 Free PMC article.
-
The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators.EMBO J. 2000 Jun 15;19(12):3080-9. doi: 10.1093/emboj/19.12.3080. EMBO J. 2000. PMID: 10856251 Free PMC article.
References
-
- Adamson A L, Kenney S C. Rescue of the Epstein-Barr virus BZLF1 mutant, Z(S186A), early gene activation defect by the BRLF1 gene product. Virology. 1998;251:187–197. - PubMed
-
- Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatful G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. - PubMed
-
- Bartkiewicz M, Gold H, Altman S. Identification and characterization of an RNA molecule that copurifies with RNase P activity from HeLa cells. Genes Dev. 1989;3:488–499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

