A conserved dileucine-containing motif in p6(gag) governs the particle association of Vpx and Vpr of simian immunodeficiency viruses SIV(mac) and SIV(agm)
- PMID: 10559313
- PMCID: PMC113050
- DOI: 10.1128/JVI.73.12.9992-9999.1999
A conserved dileucine-containing motif in p6(gag) governs the particle association of Vpx and Vpr of simian immunodeficiency viruses SIV(mac) and SIV(agm)
Abstract
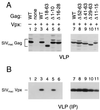
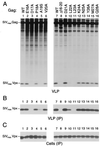
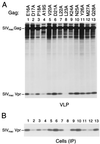
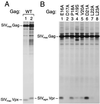
Vpr is a small accessory protein of human and simian immunodeficiency viruses (HIV and SIV) that is specifically incorporated into virions. Members of the HIV-2/SIV(sm)/SIV(mac) lineage of primate lentiviruses also incorporate a related protein designated Vpx. We previously identified a highly conserved L-X-X-L-F sequence near the C terminus of the p6 domain of the Gag precursor as the major virion association motif for HIV-1 Vpr. In the present study, we show that a different leucine-containing motif (D-X-A-X-X-L-L) in the N-terminal half of p6(gag) is required for the incorporation of SIV(mac) Vpx. Similarly, the uptake of SIV(mac) Vpr depended primarily on the D-X-A-X-X-L-L motif. SIV(mac) Vpr was unstable when expressed alone, but its intracellular steady-state levels increased significantly in the presence of wild-type Gag or of the proteasome inhibitor lactacystin. Collectively, our results indicate that the interaction with the Gag precursor via the D-X-A-X-X-L-L motif diverts SIV(mac) Vpr away from the proteasome-degradative pathway. While absent from HIV-1 p6(gag), the D-X-A-X-X-L-L motif is conserved in both the HIV-2/SIV(sm)/SIV(mac) and SIV(agm) lineages of primate lentiviruses. We found that the incorporation of SIV(agm) Vpr, like that of SIV(mac) Vpx, is absolutely dependent on the D-X-A-X-X-L-L motif, while the L-X-X-L-F motif used by HIV-1 Vpr is dispensable. The similar requirements for the incorporation of SIV(mac) Vpx and SIV(agm) Vpr provide support for their proposed common ancestry.
Figures



References
-
- Agostini I, Navarro J-M, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. - PubMed
-
- Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distinct effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu, and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. - PubMed
-
- Bouhamadan M, Xue Y N, Baudat Y, Hu B, Sire J, Pomerantz R J, Duan L-X. Diversity of HIV-1 Vpr interactions involves usage of the WXXF motif of host cell proteins. J Biol Chem. 1998;273:8009–8016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

