An epitope of the Semliki Forest virus fusion protein exposed during virus-membrane fusion
- PMID: 10559317
- PMCID: PMC113054
- DOI: 10.1128/JVI.73.12.10029-10039.1999
An epitope of the Semliki Forest virus fusion protein exposed during virus-membrane fusion
Abstract
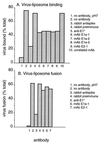
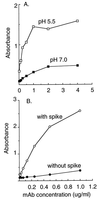
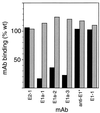
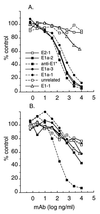
Semliki Forest virus (SFV) is an enveloped alphavirus that infects cells via a membrane fusion reaction triggered by acidic pH in the endocytic pathway. Fusion is mediated by the spike protein E1 subunit, an integral membrane protein that contains the viral fusion peptide and forms a stable homotrimer during fusion. We have characterized four monoclonal antibodies (MAbs) specific for the acid conformation of E1. These MAbs did not inhibit fusion, suggesting that they bind to an E1 region different from the fusion peptide. Competition analyses demonstrated that all four MAbs bound to spatially related sites on acid-treated virions or isolated spike proteins. To map the binding site, we selected for virus mutants resistant to one of the MAbs, E1a-1. One virus isolate, SFV 4-2, showed reduced binding of three acid-specific MAbs including E1a-1, while its binding of one acid-specific MAb as well as non-acid-specific MAbs to E1 and E2 was unchanged. The SFV 4-2 mutant was fully infectious, formed the E1 homotrimer, and had the wild-type pH dependence of infection. Sequence analysis demonstrated that the relevant mutation in SFV 4-2 was a change of E1 glycine 157 to arginine (G157R). Decreased binding of MAb E1a-1 was observed under a wide range of assay conditions, strongly suggesting that the E1 G157R mutation directly affects the MAb binding site. These data thus localize an E1 region that is normally hidden in the neutral pH structure and becomes exposed as part of the reorganization of the spike protein to its fusion-active conformation.
Figures



References
-
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. - PubMed
-
- Chatterjee, P. K., M. Vashishtha, and M. Kielian. Submitted for publication.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

